Roundtable Discussion: Mohamed Analyzes Data for Understanding ES-SCLC Treatment
During a Targeted Oncology case-based roundtable event, Mohamed K. Mohamed, MD, PhD, discussed with participants the challenges of treating extensive-stage small cell lung cancer and their views of the CASPIAN and IMpower 133 trial results.
Mohamed K. Mohamed, MD, PhD (Moderator)
Hematologist/Oncologist
Cone Health Cancer Center at Wesley Long
Greensboro, NC


MALICK: The greatest challenge that I see in ES-SCLC is after first-line treatment. Nowadays, if you have seen extensive stage and patients are started on immune therapy plus your platinum doublet, if they fail that, then there is no effective therapy that gives them long-term progression-free survival.
MOHAMED: True. This is the unmet need that we all are looking for in ES-SCLC. Any other challenges or unmet needs?
NATHWANI: I would say maybe brain metastases because this is an aggressive disease, and in extensive stage, many times it presents [one] with how to deal with brain metastases and the role of PCI [prophylactic cranial irradiation]. You know, it is still a gray zone, I think, of PCI or not. So we still need to know and learn more [about] the agents that can penetrate the blood-brain barrier.
MOHAMED: Yes, we know almost 50% or so of the patients with SCLC will end up having a brain metastasis at some point.
OSEI-BOATENG: When I have not used it, it is because they have autoimmune diseases that would preclude using immunotherapy.
MOHAMED: So a patient on immunesuppressing medication, or a patient with an autoimmune disorder—not all autoimmune disorders, but definitely severe autoimmune disorders— would not be [a patient for whom] we consider immunotherapy. Any other reason for not using immunotherapy up front?
THERTULIEN: I would add that sometimes patients present to the hospital with extensive-stage disease diagnosed in the hospital and need prompt treatment. And…in the hospital they do not allow us to use immunotherapy; that is not an inpatient drug. So we start with what we have, which is chemotherapy.
MOHAMED: Yes. It is not just your problem; it is a… problem with most of the hospitals. [They] will not allow us to use immunotherapy up front in the hospital setting.
MALICK: Our health system here firmly discourages patients [from getting] inpatient chemotherapy now. Unless we have a good case, and the patient comes in symptomatic, they do not want us to give chemotherapy [as] inpatient treatment—any sort of chemotherapy. We hardly do any, unless of course…somebody comes in with… SVCS [superior vena cava syndrome] and you give them the first cycle of chemotherapy. But generally, I do not think I have given any on an urgent case, because we [generally] use outpatient treatment.
MOHAMED: Is that the experience of others? I understand immunotherapy, but what about chemotherapy?
IRUKU: I work with Dr Mohamed, but I just moved from Colorado Springs. I used to work at the University of Colorado Health. Back there, [SCLC] just has a rapid doubling time, a high refraction rate. So I guess the tendency was to at least start the first cycle of chemotherapy without immunotherapy inpatient. And then from second cycle onward, just do everything outpatient; I guess mostly because the [prior] authorization takes time. You know, treatment is at least delayed by a week, and for infusion time, you have to get an appointment for the patient and all of that stuff. But we used to at least give chemotherapy in-house.
MOHAMED: Yes, if a patient is symptomatic, sometimes we do at least give the first cycle. If the patient is asymptomatic…not everyone diagnosed with SCLC must receive treatment at the hospital. But if it is asymptomatic from the diagnosed small cell, yes, we can treat as outpatient.

MADADI: I would consider platinum-based therapy with etoposide and immunotherapy to start out with.
MOHAMED: The patient is not currently in the hospital; would you also consider immunotherapy in addition to that?
MADADI: Yes, because [we can] start early with chemotherapy and then do a maintenance [regimen] if they are having a response with it.
MOHAMED: How would you counsel this patient regarding her treatment options? And what do you consider the most important point to communicate for a patient [with a recent diagnosis of] ES-SCLC?
MADADI: The standard has been the chemotherapy, but adding the immunotherapy, either atezolizumab [Tecentriq] or Imfinzi [durvalumab], which are both current options. These are the 2 recent drugs that have been shown to have a survival benefit compared with the standard treatment.
MOHAMED: Do you communicate the prognosis for the patient in the beginning?
MADADI: Yes, we tell them that it is extensive stage, probably not curable. Usually patients respond [well] to the treatment, but many of them relapse.… Unfortunately, when they relapse, we do not expect to get as good of a responses as…we see in the first one.

THERTULIEN: Preferentially, in the metastatic setting, I use carboplatin because it is well tolerated and, of course, [we have] the issue with the kidneys with cisplatin. I use cisplatin mainly in the adjuvant setting or, in the case of small cell, in limited stage.
MOHAMED: Is anyone still using cisplatin in the extensive stage?
MALICK: For somebody whom I normally do not see—for example, a relatively young individual with good kidney function—I do not have any problem using cisplatin rather than carboplatin. I understand the extensive stage, of course; they’re not going to cure them. So switching between cisplatin and carboplatin does not matter much. But I still use cisplatin in extensive stage, provided the patient is not elderly.
Even if they have a normal creatinine [level]—or borderline—if they have diabetes or hypertension, I tend to stay away from cisplatin. But with somebody relatively healthy, with no other comorbidities and [whose] kidney function is normal, I use cisplatin, obviously with a very close eye on the kidney function. And if I show a little bit of bump in the creatinine, I go ahead and change from cisplatin to carboplatin.
MOHAMED: Dr Malick, when you use cisplatin in this setting, do you also change the immunotherapy to fit with the cisplatin regimen, or does it matter which immunotherapy you use?
MALICK: I’ve generally used atezolizumab and not durvalumab in ES-SCLC.
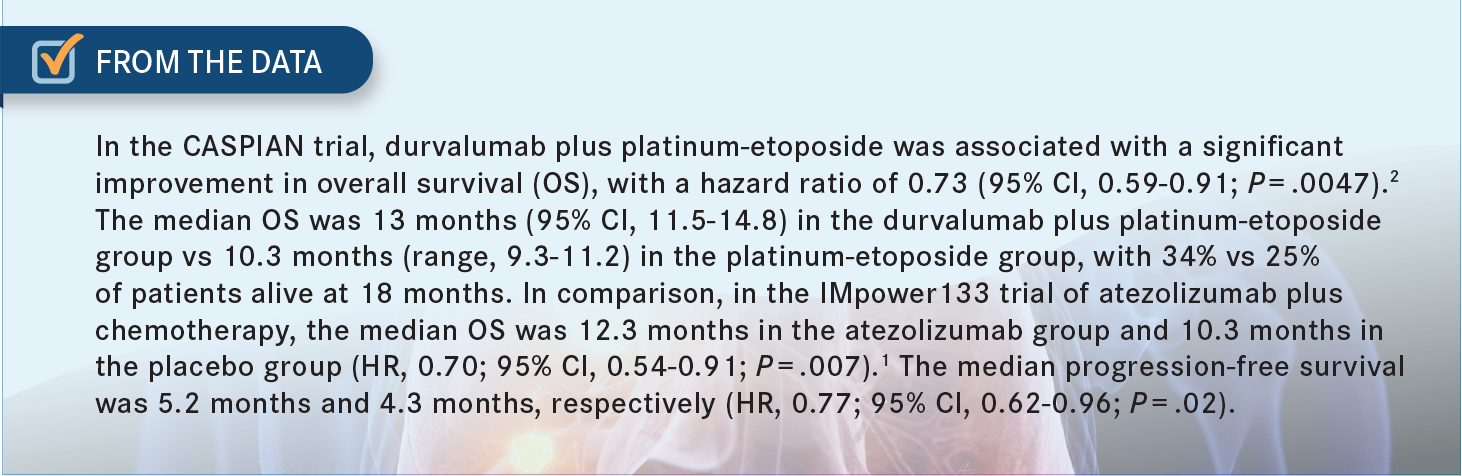
MOHAMED: In the [IMpower133 (NCT02763579)] trial, atezolizumab was given with carboplatin.1 Are you comfortable just replacing carboplatin with cisplatin and using the same immunotherapy or do you move to durvalumab because it is the one [that was studied with cisplatin] in the CASPIAN [NCT03043872] trial [From the Data].2
MALICK: If I start with one immunotherapy, I generally do not switch immunotherapies even if I am changing from cisplatin to carboplatin.


IRUKU: I have used it a few times. It is well tolerated, at least when I used it. I did not have any major toxicity. It is just that it does not work for long enough.
MOHAMED: So, no major additional toxicity compared with the chemotherapy regimen that was used?
IRUKU: Correct.
THOMAS: I have agreed with almost everything my colleagues have said. Yes, I have used it. I have had good experience with it—no additional toxicities over and above what you would expect. And just like everybody else said, it works for a while, then, unfortunately, it relapses; then you’ve got to go on to plan B.
MOHAMED: Based on the data presented for IMpower133, what is your reaction? And has this regimen affected your practice?
KATRAGADDA: I think the overall survival at 12 months and 18 months is impressive, with almost 12% improvement compared with placebo.1 I tend to use this regimen just because it was approved first and we are just used to what we are doing. That is the only reason. In the case you presented, I would be tempted to use durvalumab because of the brain metastases. I think the trial included the untreated brain metastases, and that would be my choice for this patient.2
MOHAMED: Any other opinions?
COLLINS: I have adopted this regimen, but realistically, while this is an improvement, I would like to see more improvement. It is great that we have improved survival by 2 months, but there is certainly a lot of room for more improvement.
THOMAS: I would agree with that. And occasionally a patient will look the data up themselves. One patient brought the trial [results] in to me and said, “Are you telling me this is only 8-week improvement?” And they are shocked because they think immunotherapy is a total game changer. And I agree with that.
THERTULIEN: I am not sure there is a major difference between durvalumab and atezolizumab. I think the trials were conducted differently, perhaps even in a different patient population, but in terms of brain penetration, I am not aware of truly any difference between the checkpoint inhibitors. Am I correct in this?
MOHAMED: I think you are right. Again, we are looking for more and not just what we have right now with the current immunotherapy. We are hoping for more.

MADADI: I had used durvalumab as a trial when it was approved as an alternative agent and because it has more literature on brain metastases and can be combined with cisplatin too.2 Recently, [I used it] in limited-stage SCLC, after completion of concurrent chemoradiation. And although we have no strong data for immunotherapy, because I have used a cisplatin -based regimen, I was able to get approval for durvalumab to try to do it at least for a year; however, the patient has not competed [treatment] for a year yet.
MOHAMED: For those who have not used it, [have you had] any reason for not using it?
NATHWANI: After the data came out, we just had familiarity with atezolizumab. But I guess moving forward with the brain metastases, as I talked about earlier, as we’ve seen more of that in this aggressive disease, I may [use durvalumab]. Then the combination with carboplatin: I have not used this at all stages, so that combination…does not apply to me because I think [they are] interchangeable, the cisplatin and carboplatin, in this setting. So we are stuck, at least in our practice, with carboplatin for both limited and extensive [disease].
DAROVSKY: Yes, I have been using both of the regimens and I go case by case; sometimes it depends on the clinical status of the patient. And sometimes I feel [they can be used in] the patients who are robust and who can handle a little bit more aggressive therapy, and especially if you see objective or a radiographical response, for example.
MOHAMED: But you use both of them and you are comfortable with both of them?
DAROVSKY: Yes, I do not see a big preference for one or the other.
MALICK: I have not used durvalumab in extensive stage yet. I would have no problem using it, but I do not see a difference between [the date from] those 2 studies, anyway. [Regarding] the response rate, I do not think I would rely too much on this difference. But the bottom line is that if you look at the age of survival and the progression-free survival, they are pretty much similar.2
MOHAMED: Does it make any difference that they included untreated brain metastases or that the control arm has 6 cycles of treatment vs 4?
MALICK: I am not sure with the brain metastases. But from my experience, [with] 4 cycles you are going to achieve the maximum benefit anyway. So I rarely go beyond 4 cycles, even in extensive stage, unless the patient [is] young, fit, and you see that he is getting so much great response and is almost getting to complete response on scan. But that rarely happens. So I just go 4 cycles, and they achieve maximum benefit, and then you continue immunotherapy.
MOHAMED: Does the maintenance [schedule of every] 4 weeks vs 3 weeks—I know atezolizumab can also be given every 4 weeks—make any difference [regarding] your choices?
MALICK: That is a good point. Yes, it probably would. But as far as looking at the clinical trial beyond the frequency of how often they are coming to clinic for the infusion, there is no difference.


HANNA: First, diarrhea 3 or 4 times per day is typical with a lot of chemotherapies. So first we want to make sure this is from chemotherapy or from immunotherapy. Typically, if it is 3 or 4 times per day, I would just give supportive care with maybe loperamide [Imodium] and diphenoxylate/atropine [Lomotil]. But if the diarrhea persists and does not resolve within 3 to 5 days, or if it has increased in frequency, I would think maybe this could be immune related, so I start those patients on prednisone, typically.
MOHAMED: Any other supportive care that [anyone] can provide for us here?
DAROVSKY: I was going to say, diarrhea 3 or 4 times a day sounds like a pretty casual adverse event, not very dramatic or defective. My experience is, if you watch early adverse events, early signs, I do not recall a bad experience where I had to stop immunotherapy just because of bad diarrhea. I think if you are aggressive from the beginning [with supportive care], usually it will get things under control. I do not remember even stopping immunotherapy just because of diarrhea grade 4, for example. So it is just a question of being aggressive from the beginning. This supportive care is very important.
REFERENCES
1. Horn L, Mansfield AS, Szczęsna A, et al; IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi:10.1056/NEJMoa1809064
2. Paz-Ares L, Dvorkin M, Chen Y, et al; CASPIAN Investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. doi:10.1016/ S0140-6736(19)32222-6













