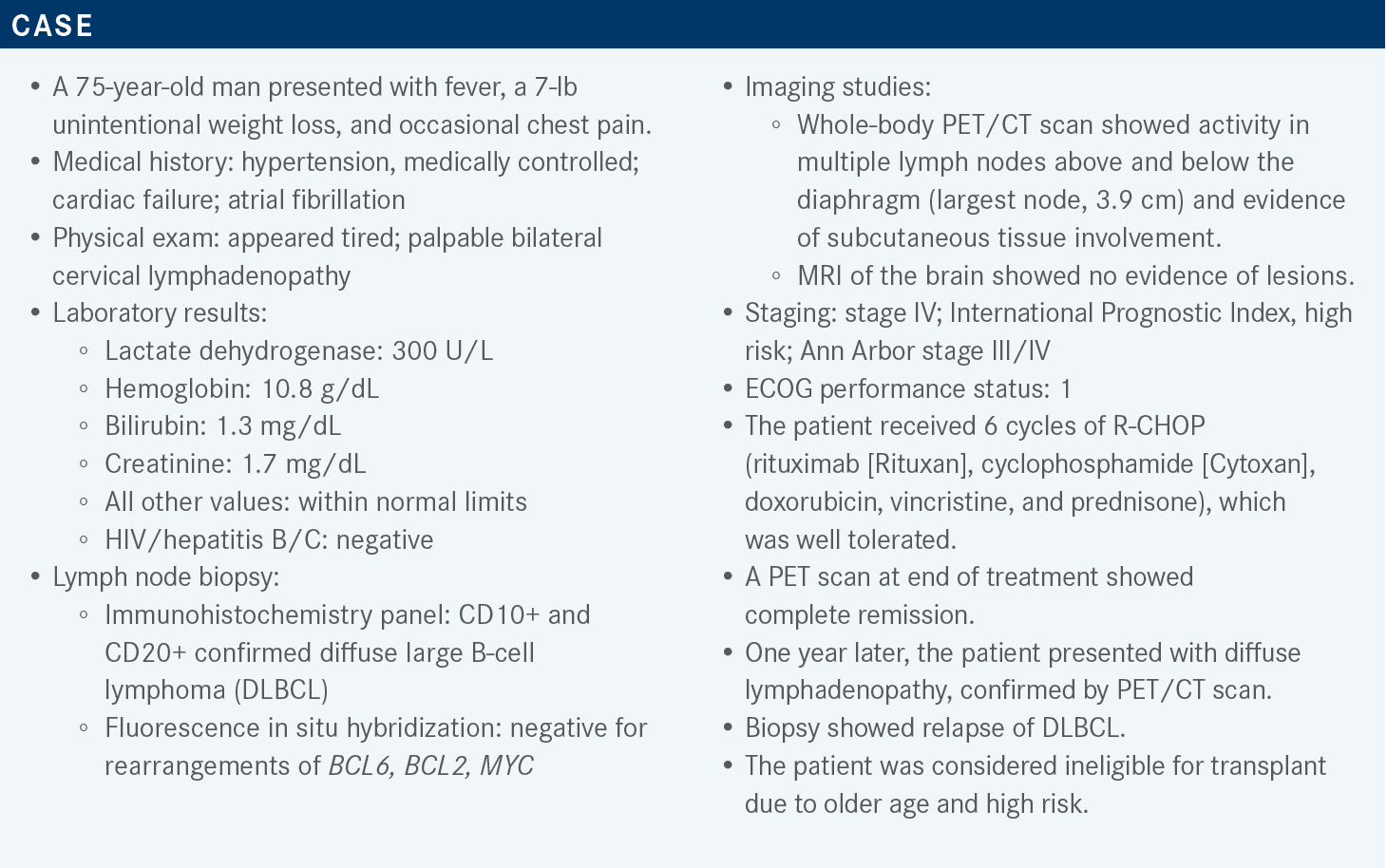
Hamadani Discusses Data in Favor of Systemic Therapies for DLBCL
During a Targeted Oncology case-based roundtable discussion, Mehdi H. Hamadani, MD, discussed systemic therapy options for relapsed/refractory diffuse large B-cell lymphoma.
Mehdi H. Hamadani, MD
Professor of Internal Medicine
Medical College of Wisconsin
Director, Adult Blood and Marrow Transplant Program
Froedtert Hospital
Milwaukee, WI

Targeted OncologyTM: In the relapse and refractory setting, what proportion of patients receive CAR (chimeric antigen receptor) T-cell therapy?
HAMADANI: I think 10% to 25% is a very realistic estimate of [the proportion of] patients who actually receive CAR T-cell therapy. I think if somebody considers this patient a candidate for CAR T-cell therapy, [that would] not [be] unreasonable, depending on the comorbidities and the performance status. [Those things would be assessed] on a case-by-case basis. In our program we probably would consider [CAR T-cell therapy for this patient] as well.

What data support the use of polatuzumab vedotin (Polivy) plus bendamustine/rituximab (BR)?
This combination was provided accelerated approval1 based on results of a randomized phase 2 trial [NCT02257567]. That obviously means that this drug needs a confirmatory trial to confirm these benefits. The trial included patients above the age of 18 years with relapsed or refractory DLBCL. These patients needed to have [had] at least 1 prior line of therapy, and these patients could not be transplant eligible or have had a prior allogenic transplant or transformed lymphoma. It is important to keep this in mind.
Although they did not exclude double-[hit] lymphomas, the trial did not enroll any patients with double-[hit] lymphoma. The patients were randomly assigned to receive either the standard of care BR or the experimental therapy [polatuzumab vedotin plus BR]. Polatuzumab was given at a dosing schedule of 1.8 mg/kg, intravenously, every 21 days for 6 cycles.2
The median [number of] prior lines of therapy was 2, and most of these patients [approximately 80%-90%] had advanced-stage disease. About one-third to 40% of these patients had the germinal center B-cell [GCB] subtype of DLBCL, and [the patients in this study had a median age of approximately 70 years].
The primary end point of the study was end-of-treatment objective response rate [ORR]. The ORR by Lugano criteria was about 18% in the comparator arm and was significantly higher [45%] in the investigational arm [polatuzumab plus BR].2 That trial also had an expansion cohort of about 100 patients, and even in the expansion cohort, the ORR was confirmed at 41.5%.3
Progression-free survival [PFS] was longer in patients in the investigational arm than in the comparator arm [median PFS, 9.2 months (95% CI, 6.0-13.9) compared with 3.7 months (95% CI, 2.1-4.5), respectively]. The median duration of response [DOR] was comparable [between the arms], 10.9 months for the investigational arm and 10.6 months for comparator arm.3 Of course, when you look at DOR, you are looking only at those patients who responded to treatment.
The median overall survival [OS] was about a year [12.4 months; 95% CI, 9-32] with polatuzumab plus BR, and 4.7 months [95% CI, 3.7-8.3] with only BR.3 We see [this as] a signal that this triplet regimen was making the patients live longer as well.
Are there notable toxicities with this treatment?
Polatuzumab is basically glorified brentuximab vedotin [Adcetris], as it has the same immunotoxin. [There was] a little bit more grade 3 and grade 4 neutropenia with polatuzumab plus BR [46.2%] than with BR alone [33.3%]. Obviously, any bendamustine-based regimen is going to cause myelosuppression. Febrile neutropenia was about the same in both groups, at [approximately] 10%. Neuropathy is obviously noteworthy because the combination of bendamustine and rituximab generally doesn’t cause neuropathy, although bendamustine can, a little bit.
Peripheral neuropathy was seen in about 40% of the patients in the investigational arm, and it was seen in only [approximately] 7% of the patients in the comparator arm, so neuropathy is a distinction between the regimens. More patients in the investigational arm than in the comparator arm discontinued therapy because of adverse events [33% vs 10%, respectively]. But the dose reductions were relatively comparable, occurring in 5% of the patients in the investigational arm and in 13% of the patients in the comparator arm.2
What is the mechanism of action of tafasitamab (Monjuvi) plus lenalidomide (Revlimid)?
Tafasitamab is an Fc-[enhanced], naked, anti-CD19 monoclonal antibody, and there is biological rationale to combine it with lenalidomide. Lenalidomide has some important T-cell– and natural killer [NK]-cell–activating properties. Lenalidomide is directly cytotoxic to lymphoma cells. But in addition to [having] direct antilymphoma activity, [lenalidomide] also [has the ability to] augment immune responses mediated by T and NK cells, which augments the antibody-dependent cytotoxicity and phagocytosis of tafasitamab. [This was] the biological rationale of combining a monoclonal antibody with lenalidomide, [as was done in the phase 2 L-MIND study (NCT02399085)].
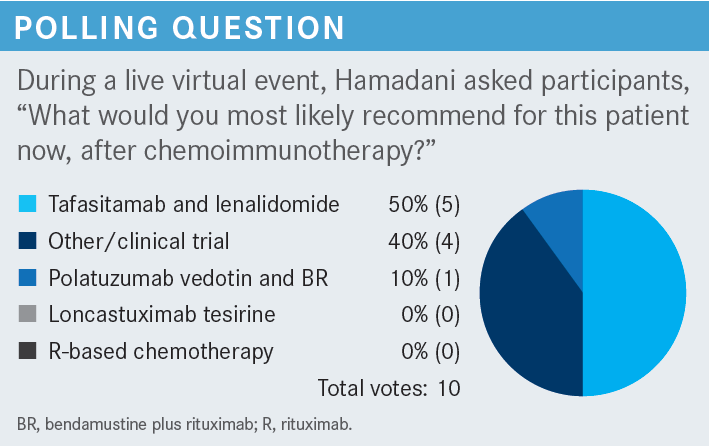
What data support the use of loncastuximab tesirine (Zynlonta)?
[The LOTIS-2 trial (NCT03589469) provided those data.] The type of patients who went onto this trial was interesting. [In comparison with the trials mentioned above],2,4 this trial arguably had more heavily pretreated patients. The median [number of] prior lines of therapy in this trial was 3. Unlike the study of polatuzumab plus BR,2 this trial did not exclude patients with transformed lymphoma. It [also] did not exclude double hit and triple hit patients. In fact, 15 double-hit and triple-hit patients were on this study.
The cell of origin was equally distributed [33% GCB, 16% non-GCB, and 51% other]. It is a noteworthy point that 13 patients on this trial had received CD19-directed CAR [T-cell] therapies, failed [those] therapies, and then came on this trial. And this trial also had a good number of patients who were refractory to frontline therapies, about 20%.5 So we can go out on a limb and say that maybe the trial did not have an inclusion bias to bring in lower-risk patients.
[When considering the] efficacy [of this therapy], remember that tafasitamab plus lenalidomide is a 2-drug combination and polatuzumab plus BR is a 3-drug combination. This [study explored] a single agent…and the overall response rate was 48% [95% CI, 39.9%-56.7%], with 24% of the patients achieving a complete response [CR] and 24% of the patients achieving a partial response. These responses were obtained quite briskly. The median time to first response was 41 days [Interquartile range, 38-44]. This basically means that you give 2 cycles, 21 days apart, [and then] you do a PET scan, and at your first assessment you would see a response. Most responses happened after the second cycle.5,6
Of the patients who responded, the median DOR was [10.3] months [95% CI, 6.9-not estimable].7 The median DOR for patients who achieved a CR was not reached at the last follow-up in the March 2021 update.6 So the CRs tended to stick around for a while.
How does this drug perform in high-risk patients?
The double hit and triple hit patients responded [overall response rate, 33.3%; 95% CI, 11.8%-61.6%], and the transformed patients with lymphoma, who are high-risk, of course, had response rates comparable to those patients with de novo DLBCL [44.8%; 95% CI, 26.4%-64.3% vs 49.1%; 95% CI, 39.7%-58.6%, respectively].
There was no difference [in response rate] between activated B-cell and GCB subtypes [47.8%; 95% CI, 26.8%-69.4% vs 54.2%; 95% CI, 39.2%-68.6%, respectively]. Almost half of the patients with high-grade B-cell lymphoma responded [45.5%; 95% CI, 16.7%-76.6%], and most of those patients are double-hit cases. Among patients who had primary refractory disease, about 38% [95% CI, 20.7%-57.7%] responded.
The response rates were comparable between patients who had received a prior transplant and those who had not [58.3%; 95% CI, 36.6%-77.9% vs 46.3%; 95% CI, 37.2%-55.6%, respectively]. And interestingly, the patients who had received prior CAR T-cell therapy had a [46.2%] response rate [95% CI, 19.2%-74.9%]. Patients who didn’t have prior CAR T-cell therapy had a [48.5%] response rate [95% CI, 39.7%-57.3%].7 And, intuitively, most of these CAR T-cell therapies would have been CD19 directed. So it was a nice thread of activity.
PFS includes responders and nonresponders [median PFS was 5.1 months (95% CI, 2.9-8.3) at the August 2020 cutoff]. The median OS of all-comers in third line and beyond was about 10 months [95% CI, 6.9-11.3]. Some of the patients who responded came off the trial and went on to receive a stem cell transplant, either an autologous or an allogenic transplant, with curative intent. Some of these patients received CD19-directed CAR T-cell therapies after failing loncastuximab, and these patients responded to the CD19-directed CAR T-cell therapies as well [overall response rate, 46.7%].7
[Among the patients with] high-grade B-cell lymphoma, the double-hit and triple-hit [patients], [45.5%] of the patients responded.8 Interestingly, all the double-hit patients who responded were CRs, so when [a therapy] works in that setting, maybe [we can say that] it works well. [Among the] patients who got this drug after CAR T-cell therapy failed, CD19 was preserved on the cell surface, and they could come on the LOTIS-2 trial. The response rate [of those patients], even after CAR T-cell therapy failed, was [approximately] 45%.9,10
Are there safety concerns with this therapy?
In terms of safety, [loncastuximab] is an interesting drug. One of the adverse events [seen in this study was] elevated γ-glutamyl transferase [GGT].5 Occasionally you will see [aspartate aminotransferase and alanine aminotransferase] elevations. It is recommended to monitor [that].
If you see a lot of elevation in GGT [levels], hold the drug until it improves. There is a little bit of [a] myelosuppression signal [with this regimen], but grade 3 or grade 4 neutropenia and thrombocytopenia [are] not [very] common. Fluid accumulation, including peripheral edema and occasionally pleural effusions, needs to be monitored.5
REFERENCES
1. FDA approves first chemoimmunotherapy regimen for patients with relapsed or refractory diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed June 18, 2022. https://bit.ly/2EXFLOT
2. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
3. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi:10.1182/bloodadvances.2021005794
4. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
5. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/S1470-2045(21)00139-X
6. Caimi P, Ai WZ, Alderuccio JP, et al. Duration of response to loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma by demographic and clinical characteristics: subgroup analyses from LOTIS-2. J Clin Oncol. 2021;39(suppl 15):7546. doi:10.1200/JCO.2021.39.15_suppl.7546
7. Caimi PF, Ai WZ, Alderuccio JP, et al. Efficacy and safety of loncastuximab tesirine (ADCT-402) in relapsed/refractory diffuse large B-cell lymphoma. Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 5-8, 2020; virtual. Abstract 1183. Accessed June 19, 2022. https://bit.ly/3yltuPh
8. Alderuccio JP, Ai WZ, Radford J, et al. Clinical characteristics and responses of patients with relapsed or refractory high-grade B-cell lymphoma treated with loncastuximab tesirine in the Lotis-2 clinical trial. Presented at: 63rd American Society of Hematology Annual Meeting and Exposition; December 9-16, 2021; Atlanta, GA. Abstract 3575. Accessed June 19, 2022. https://bit.ly/3I26fy8
9. Caimi PF, Ardeshna KM, Reid E, et al. The anti-CD19 antibody-drug conjugate loncastuximab tesirine achieved responses in patients with diffuse large B-cell lymphoma who relapsed after anti-CD19 CAR T-cell therapy. Presented at: 63rd American Society of Hematology Annual Meeting and Exposition; December 9-16, 2021; Atlanta, GA. Abstract 2489. Accessed June 19, 2022. https://bit.ly/3bzyesu
10. Caimi PF, Ardeshna KM, Reid E, et al. The antiCD19 antibody drug immunoconjugate loncastuximab achieves responses in DLBCL relapsing after antiCD19 CAR-T cell therapy. Clin Lymphoma Myeloma Leuk. 2022;22(5):e335-e339. doi:10.1016/j.clml.2021.11.005