Roundtable Discussion: Tejwani Considers Key Factors for Choosing Systemic Therapy for Metastatic CSPC
During a Targeted Oncology case-based roundtable event, Sheela Tejwani, MD, discussed therapy options for a patient with metastatic castration-sensitive prostate cancer.
Sheela Tejwani, MD
Medical Oncologist
Henry Ford Cancer Institute
Henry Ford Health System
Detroit, MI


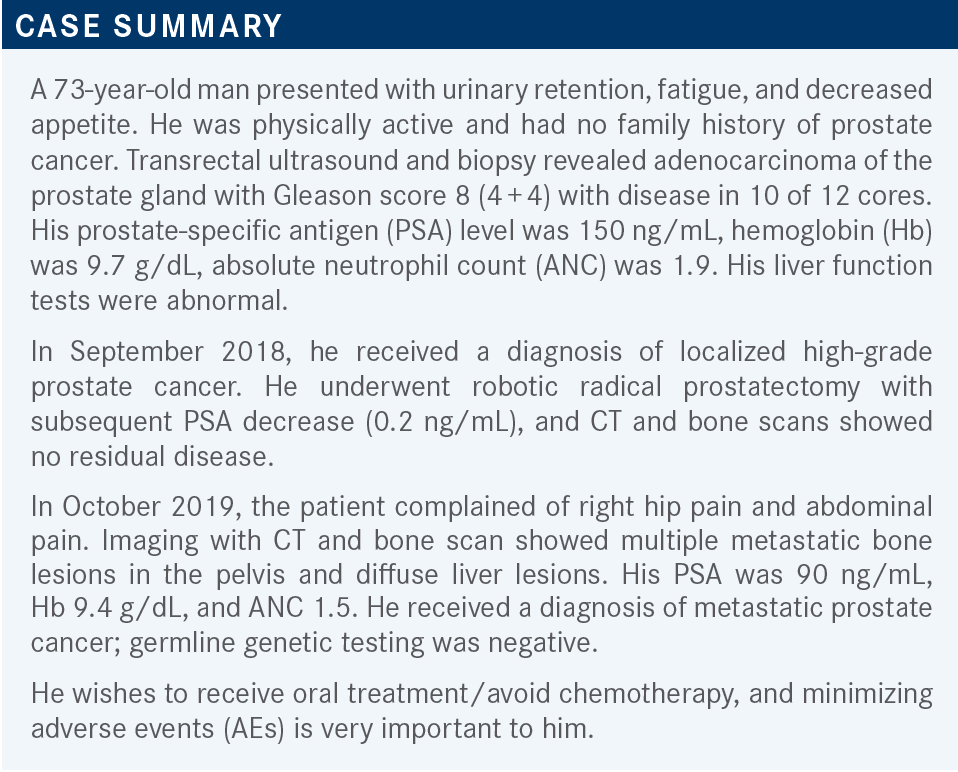
BALLOUZ: In general, I do not [use disease volume]. I start with the double hormonal treatment and see what happens. Usually if they respond, they respond quickly, in my experience.
BILBEISI: If there was a low amount of tumor burden— say, for example, the patient had low amounts of bone metastases and that was it—I probably would have just done ADT alone.
TEJWANI: But this patient did have liver lesions.
BILBEISI: I know, but you asked how my choice would differ if there was less tumor burden. If it had been something else like [only] a bone metastasis, I probably would have chosen ADT alone, but I chose ADT with enzalutamide [Xtandi] for this patient.
TEJWANI: Sure. I have used ADT alone in older patient populations because of their comorbid conditions or the age factor. I have an 82-year-old who has multiple comorbidities and he said, “If my PSAs are good, I do not want to go on the combination.” But the combination is recommended by the NCCN [National Comprehensive Cancer Network] guidelines.1 So for few bone metastases, age factor, and comorbid conditions, ADT alone could be an answer for those individuals.
BLOOM: Yes, this patient only wanted to take oral therapy. That pretty much excludes docetaxel [Taxotere] from consideration, so I picked ADT and enzalutamide as probably the most effective oral combination in this gentleman.
TEJWANI: Dr Bloom, do you differentiate when to choose abiraterone [Zytiga] vs enzalutamide?
BLOOM: No. My impression is there are no head-to-head [comparisons].
TEJWANI: Yes, that is true. But let us say you have a patient with diabetes.
BLOOM: My impression is that enzalutamide is a little bit stronger but has a little more toxicity. So in people who are robust, I tend to use it. In patients who are slightly less robust, I tend to use abiraterone. But that’s as good as I can tell you, because I don’t know that I’m right.
TEJWANI: I agree with you, mostly. I do differentiate. So say if you have a brittle patient with diabetes who’s already on insulin or multiple diabetic medications, then you must monitor hypokalemia and hypotension more closely, so for patients like that, I prefer to go with enzalutamide. Of course, you must do the exclusion to make sure they have not had any seizure disorder, they have not had any automobile accident with brain trauma, or any stroke, and things like that. I do agree with you, my first go-to is enzalutamide. It’s easy to take. There’s not too much monitoring involved with enzalutamide.
BLOOM: But there’s a little more dysphoria associated with that.
HASSAN: I agree with the ADT plus enzalutamide. The only thing I would add is bisphosphonates, and I don’t know how bad this patient has bone disease in terms of pain management and if they would benefit from palliative radiation if needed.
TEJWANI: If I may say, this is a patient with castration-sensitive prostate cancer [CSPC]. If a patient has osteoporosis, only then do we offer bisphosphonates. It is only approved in castration-resistant prostate cancer [CRPC].

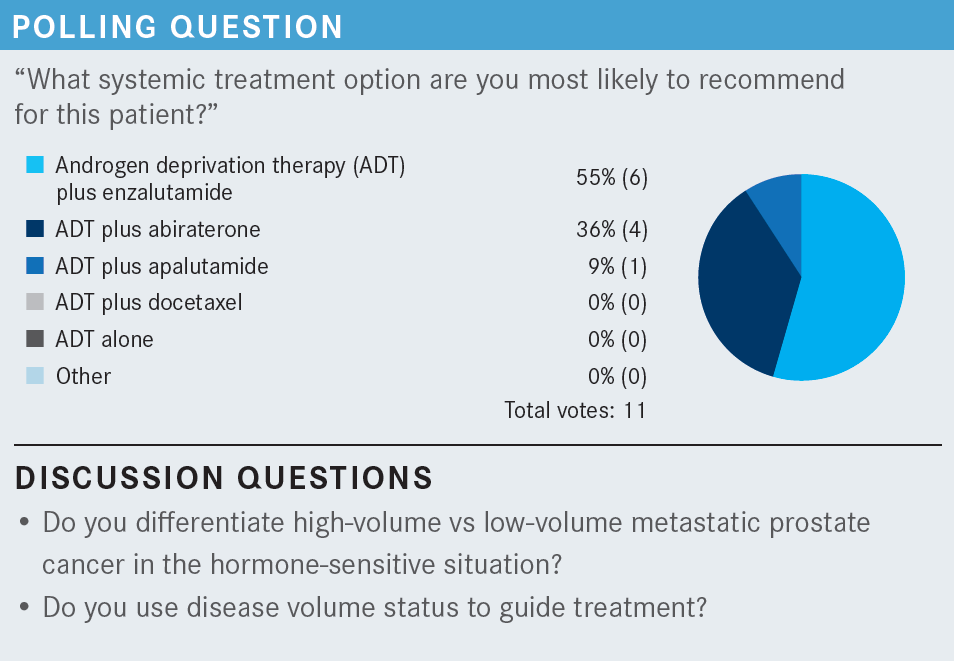
JASTI: I do not specifically go by the PSA level. I look at the PSA velocity, but I also look at the Gleason score and the volume of the disease in deciding whether to use dual therapy.
TEJWANI: I agree with you because I also don’t go with the PSA level. We have had patients with 1000 ng/mL or more of PSA but bone-only disease, no liver involvement. Lung involvement is rare, but we don’t go by the PSA.
KHAN: In terms of PSA, obviously it does not guide treatment selection. However, if there is large-volume disease with a high PSA level and shortened time from the diagnosis to the development of metastatic disease, I think those are all real clinical concerns in terms of how quickly this disease may respond and eventually may progress as well. I think it is just to guide us with the history or the course of the disease. I think that way it is helpful, but to be honest, it is not of any help for deciding what treatment option to use.
TEJWANI: Right. The doubling time you are referring to is more for CRPC biochemical recurrence. [Based on the ARAMIS [NCT02200614] and PROSPER [NCT02003924] trials, the FDA approved] darolutamide [Nubeqa] and enzalutamide, respectively.2,3
The doubling time does matter, but in the setting of stage IV CSPC, I think I personally will not just lean on the PSA level, but I will lean more toward volume of cancer like visceral involvement. Even if there are 1 or 2 bone lesions, that qualifies for high volume. Now, this patient who was discussed did not want to go on chemotherapy. But there are patients who do.
I have recently had a new patient with liver lesions and very few bone lesions. He’s a priest, and he said, “How long will I have to be on the hormonal agents?” I said, “At least 2 to 3 years,” so he chose chemotherapy and he qualified for high-volume disease, so he started docetaxel last week. I think it depends on the patient’s preference as well, and a lot of men are not keen to go on chemotherapy.
KHAN: Certainly, in a case like this, if there was no preference for oral chemotherapy, I would have gone straight to docetaxel because of the high volume of disease.

TEJWANI: There are treatment-related factors with the different drugs we have talked about, then disease-related factors and patient-related factors. In patient-related factors, we have age, frailty, comorbid conditions, family, and social history. Anticipated [adherence] is very important, and convenience. With the oral drugs, we want [an adherent] patient. The disease-related factors have to do with the volume of disease or the tumor burden, PSA level, Gleason score, site of disease involvement, symptoms, and molecular test results.
The treatment-related factors are the efficacy data. I think Dr Bloom mentioned that there are no head-to-head comparisons between docetaxel, enzalutamide, and abiraterone. These are all based on the trials, but there are no direct trials comparing these to determine which one is a better option. The toxicity data also play a part in choosing which option you go with—availability, cost, and logistics, too. As you know, most of these prostate cancer drugs are quite expensive, anywhere between $10,000 and $15,000 a month, and the co-pay costs are high, too, so there is financial toxicity. Also, comedications and drug interactions count. Does anyone use these criteria to select?
LIU: I think you touched on most of them. There are no head-to-head comparison studies, so it depends on comfort level of each physician and the AE-related issues. Regarding the tumor burden, I agree tumor burden is probably one of the most important selection criteria up front. For the PSA level I agree with everybody else. I don’t use that as a selection criterion because it can be misleading. Some patients have a huge burden of disease, but their PSAs are quite low.
TEJWANI: Absolutely. How about the molecular test results?
LIU: In this setting, probably not too much. Maybe later it will have an effect, but this is the first-line treatment for hormone-sensitive disease.
TEJWANI: I agree with that.

TEJWANI: Of course, they mentioned in our patient’s history that he preferred no chemotherapy and wanted oral agents.
MCGRATH: For me, if they don’t want chemotherapy, then it’s just a question of which oral therapy to use. I decide myself between the 3 which I think is going to be better for them. But of course, I first discuss about just the ADT alone, oral, or IV [intravenous], just to see where they fall at in that category, but for the orals, I don’t leave it up to them.
TEJWANI: Say before you walk in the consult room, you had in mind this patient has liver metastasis and you were going to offer the docetaxel. When you start the conversation with the patient, do you talk them into chemotherapy because of liver metastasis? How do you approach the conversation?
MCGRATH: They usually know if they’re totally against IV to begin with, so that conversation is short. It’s not too often that we have a prolonged conversation about that because the majority of patients don’t want IV chemotherapy, especially once they know there’s an oral one. They [say,] “Maybe later, but right now I just want the orals,” so it’s a short-lived discussion.
TEJWANI: Do you go into conversation about the longevity of oral drugs vs IV treatments?
MCGRATH: Yes, that used to sway me quite a bit, but it doesn’t seem to sway the patients.
TEJWANI: You’re totally right. Dr Patel, any thoughts on this?
PATEL: We’ve already reviewed that age and performance status are highly important for these patients regardless of what treatments you’re going to recommend. Then, of course, I am looking at the tumor burden. If there’s a lot of tumor burden, then I’m leaning toward chemotherapy. However, I do use the NCCN guidelines heavily, especially for my younger patients, so I let them see it and then we talk briefly about the AEs. For me, if there is a lot of seizure history or neurologic history, I stay away from enzalutamide but fall on abiraterone. I don’t see a huge difference except for that. Most of the AEs I find are very similar. But yes, performance status and age are what I highly consider. It is still a 1-hour consult, so we go over all the AEs.
TEJWANI: How much do you weigh in on the patient’s preferences?
PATEL: Quite a bit because insurance is always high on their minds. As you noted, they are very expensive drugs. Docetaxel is 6 cycles, and they can manage this because most of it gets paid [by insurance] and it is not long term, so I lean toward chemotherapy for high tumor burden, but again, ultimately, it’s the patient’s choice.
SHEQWARA: We’re getting more and more data collection and we’re focusing more on what patients want, how they feel, and what their QOL is. I would consider patient-reported outcome data as a significant decision-making point, as well as the patient preferences, quite a bit. Goals of therapy I think, as all my colleagues have mentioned, are about how to pick between the 2 oral hormonal therapies and when to pick chemotherapy and kind of to tailor therapy based on all these factors that were mentioned. So quite a bit, we do involve these points in decision-making.

TEJWANI: I’m sure in other institutions, you all get patients who recurred and have metastatic disease and the urologist started them either on bicalutamide [Casodex] or leuprolide [Lupron]. Then they arrange for the medical oncology consult. How do you counsel patients that we need something more besides the ADT?
BALLOUZ: If the patient has extensive disease, I’m going to talk to him about more aggressive treatments.
TEJWANI: So how do you have the conversation with the patient?
BALLOUZ: I present the data and what my experience is and what I know about the natural history of the disease and what the benefit is of each approach. Then I tell them what I recommend. I usually make the final decision. I tell them that if we do this, probably their outcome will be better. I touch on the other options, but I decide because patients don’t know. It’s not fair to ask them what they think. I must make the decision, so I just tell them what I think is the best for them and I do it.
TEJWANI: In your practice, can you sway them? I have maybe 80% who I’m able to say we need something more and they will agree, but 20% will still give resistance. Why do I have to do this? You showed me the data, but I want to see the PSA levels. Do you get into those kinds of problems?
BALLOUZ: Yes, I do. Especially in the older patient population, I see more resistance. The younger population is more agreeable. The older ones say, “Leave us alone, we’re good and happy.” I [somewhat] agree. The younger ones want everything under the sun because they want to feel better and they want to live longer, so it’s easier to get those medications to the younger people.
TEJWANI: Yes, I would agree with that. Older patients tend to have more resistance, especially between ages 78 and 85, in whom we are getting a lot of metastatic CSPC. So this kind of discussion is done with them.

TEJWANI: We want to discuss your perspective on the tolerability of apalutamide and ADT vs other combinations. Dr Khan, do you want to comment?
KHAN: I believe that the TITAN study was a good study in terms of progression-free survival [PFS], which [along with OS] was a primary end point of the study [From The Data4].4,5
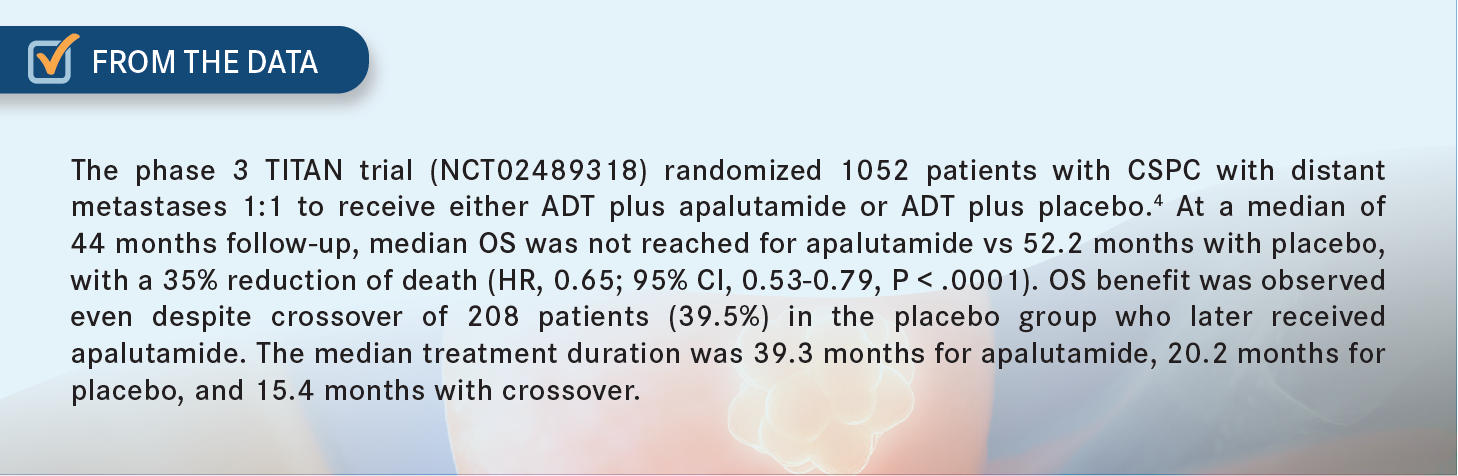
Since enzalutamide is approved in both CSPC and CRPC settings, we typically have more experience with enzalutamide than with apalutamide. I still feel more comfortable using the enzalutamide because of its favorable toxicity profile, as well as the data we have for both castrate-sensitive and resistant disease.
BALLOUZ: Of course, the AEs to monitor are completely different between the 2. I think we all know how to follow those patients, either on docetaxel or other treatment. What triggers my changing therapy is usually increased PSA, worsening metastatic disease, and more symptoms. I think we all do that in our practice.
TEJWANI: Absolutely. Docetaxel for sure requires much more close monitoring. You must see the patient every 3 weeks, through the 6 cycles, and you monitor for febrile neutropenia, ANC, and nail changes, so it is also not [a well-tolerated] drug. With enzalutamide, initially I see them maybe once a month for 1 or 2 times, and after that, I put them on every 3 months. With abiraterone and prednisone, we monitor for the laboratory abnormalities.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 3.2022. Accessed June 13, 2021. https://bit.ly/3xLq3SP
2. FDA approves enzalutamide for castration-resistant prostate cancer. FDA. July 16, 2018. Accessed June 13, 2022. https://bit.ly/3mK8s7w
3. FDA approves darolutamide for non-metastatic castration-resistant prostate cancer. FDA. July 31, 2019. Accessed June 13, 2022. https://bit.ly/3mOdXlj
4. Chi KN, Agarwal N, Bjartell A, TITAN Investigators, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. doi:10.1056/NEJMoa1903307
5. Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN Study. J Clin Oncol. 2021;39(20):2294-2303. doi:10.1200/JCO.20.03488
















