Anderson Reviews Data for Belantamab Mafodotin as Later-Line Therapy for Multiple Myeloma
During a Targeted Oncology case-based roundtable event, Larry D. Anderson, MD, PhD, discussed the results of the DREAMM-2 trial and management of belantamab mafodotin for patients with multiple myeloma.
Larry D. Anderson, MD, PhD
Associate Professor
Division of Hematology/Oncology
Department of Internal Medicine
UT Southwestern Medical Center
Dallas, TX

Targeted OncologyTM: Can you discuss BCMA (B-cell maturation antigen)–targeted therapies for relapsed/refractory multiple myeloma?
ANDERSON: Before we get into the therapeutics and data, I’ll just mention a little bit of background. BCMA is a protein receptor found on all plasma cells, and it’s overexpressed on myeloma cells. Expression goes up with progression of disease from MGUS [monoclonal gammopathy of undetermined significance] to smoldering to active, and then with increased burden of disease. It has 2 natural ligands, APRIL [a proliferation-inducing ligand] and BAFF [B-cell activating factor]. It can also be cleaved from the surface to form the soluble BCMA, which could have impact on certain therapeutics, and potentially could also serve as a marker for myeloma response and progression.
In order to harness this as a target, there are several different methods that have been used. One of the methods is an antibody-drug conjugate. That is essentially an antibody that has been bound to a toxic payload to target that therapeutic directly to the plasma cells without having nonspecific toxicity of that therapeutic all over the body.
Chimeric antigen receptor [CAR] T cells have essentially a mixed receptor that has an end of an antibody that binds BCMA and a tail end of a T-cell receptor joined to it to signal the T cells and force them to kill the myeloma. Other therapeutics [include] bispecific antibodies. There are many trials looking at bispecifics, which have half of the molecule binding to BCMA and the other half binding to CD3 to redirect those T cells to kill myeloma.
What are the preferred and recommended therapies for a patient who has progressed following several lines of treatment?
As for what to do with someone who’s had a couple of prior lines of therapies for myeloma, there’s an alphabet soup of combinations of therapies. According to the NCCN [National Comprehensive Cancer Network] guidelines, most of it is to be tailored to what the patient has already had, what didn’t work, and what their preferences are. But there are a lot of different combinations available, which is a blessing.1
Once we get to patients who have had more than 3 prior therapies, we do start to drop off on the number of options available. The tricky thing is, what do we call prior therapies vs lines of therapy? That’s a little controversial and debatable. For example, if they’ve had more than 3 prior therapies, they would be a candidate for belantamab mafodotin [Blenrep]—although, technically, the FDA labels for both of the CAR T-cell products I’ll mention require 4 prior lines of therapy—not just therapies, but lines—so that’s a little tricky. And then selinexor [Xpovio] is indicated once they’ve had a couple different IMiDs [immunomodulatory drugs], a proteasome inhibitor [PI], and an anti-CD38 antibody.1
The EHA-ESMO [European Hematology Association and European Society for Medical Oncology] guidelines strongly favor—for the patients who had at least 1 or 2 prior lines of therapy but are triple refractory to IMiD, PI, and anti-CD38—belantamab, an antibody-drug conjugate, as the next line of therapy.2 However, if [patients are] just lenalidomide or bortezomib refractory, we’re mainly going to be adding in a monoclonal antibody combination. And if they’re PI and lenalidomide refractory, we’re going to be switching classes and adding an antibody. There are a lot of different options for these patients.
Then we also have the IMWG, the International Myeloma Working Group.3 Essentially, PI and antibody combinations are preferred. [Other options are] selinexor and belantamab and high-dose, or aggressive, inpatient chemotherapy such as VdT-PACE [bortezomib, dexamethasone, thalidomide (Thalomid), cisplatin, doxorubicin, cyclophosphamide, and etoposide (Etopophos)]. If a patient is rapidly progressing and needs bridging to something else, then [we use] CAR T-cell therapy once these patients have had 4 prior lines.
Can you discuss the clinical data that led to the approval of belantamab mafodotin?
This medication, belantamab mafodotin, was approved by the FDA in August 2020 based on the DREAMM-2 study [NCT03525678].4,5 The FDA label requires 4 prior therapies, including an IMiD, a PI, and a CD38. The recommended dose, or the FDA-label dose, is 2.5 mg/kg [intravenously] over 30 minutes every 3 weeks,6 although I will note that in my practice, I often start out at every 4 weeks just to try to minimize the ocular toxicity.
The DREAMM-2 study was a phase 2 trial that was randomized between 2 different doses of belantamab: 2.5 mg/kg and 3.4 mg/kg.5 It was based on [the DREAMM-1] phase 1 trial [NCT02064387], which showed good activity at both levels but was trying to tease out which might have the highest efficacy-to-toxicity ratio. In this study, patients had at least 3 prior lines of therapy, and the primary outcome was overall response rate [ORR], but they were not exposed to prior BCMA therapies in this study. They were exposed to prior PI, IMiD, and CD38.
There were 97 patients enrolled in the DREAMM-2, and median age was 65.5,7 The median number of prior therapies was 7—I just wanted to point that out. These were heavily refractory patients, with a range of 3 to 21 prior lines of therapy. Many of these patients did have high-risk chromosomes such as 14;16, 4;14, 17p, etc. The median follow-up [according to results published in the journal] Cancer was 12.4 months, and the patients had received between 1 and 11 cycles.7
For this heavily refractory population, the ORR was approximately 31% [97.5% CI, 21.7%-43.6%], including a minority with complete responses, a little bit more with a VGPR at 11%, and 13% with a partial response.7 But, of note, some additional patients had a minor response, and a little over [25%] of the patients also had stable disease, so you could also [observe a] combined clinical benefit that was higher than the ORR. The median duration of that remission was approximately 11 months. Also, the responses deepened over time, so if they were able to stay on therapy, [efficacy] could often improve down the road. The median progression-free survival was approximately 2.8 months [95% CI, 1.6-3.6]. Median overall survival was 13.7 months [95% CI, 9.9–not reached]. It did work in patients with high-risk chromosomes and renal impairment. But it didn’t work as well in patients with extramedullary disease.
As far as toxicity, the main 2 toxicities to note were ocular toxicity, or keratopathy, and thrombocytopenia, which happened in the majority of patients.7 Many of these were low grade, but [there was] a fair amount of grade 3 or higher keratopathy, and approximately 22% experienced higher-grade thrombocytopenia as well; [there also were] some other cytopenias.
What are the ocular toxicities associated with belantamab mafodotin, and how can they be managed?
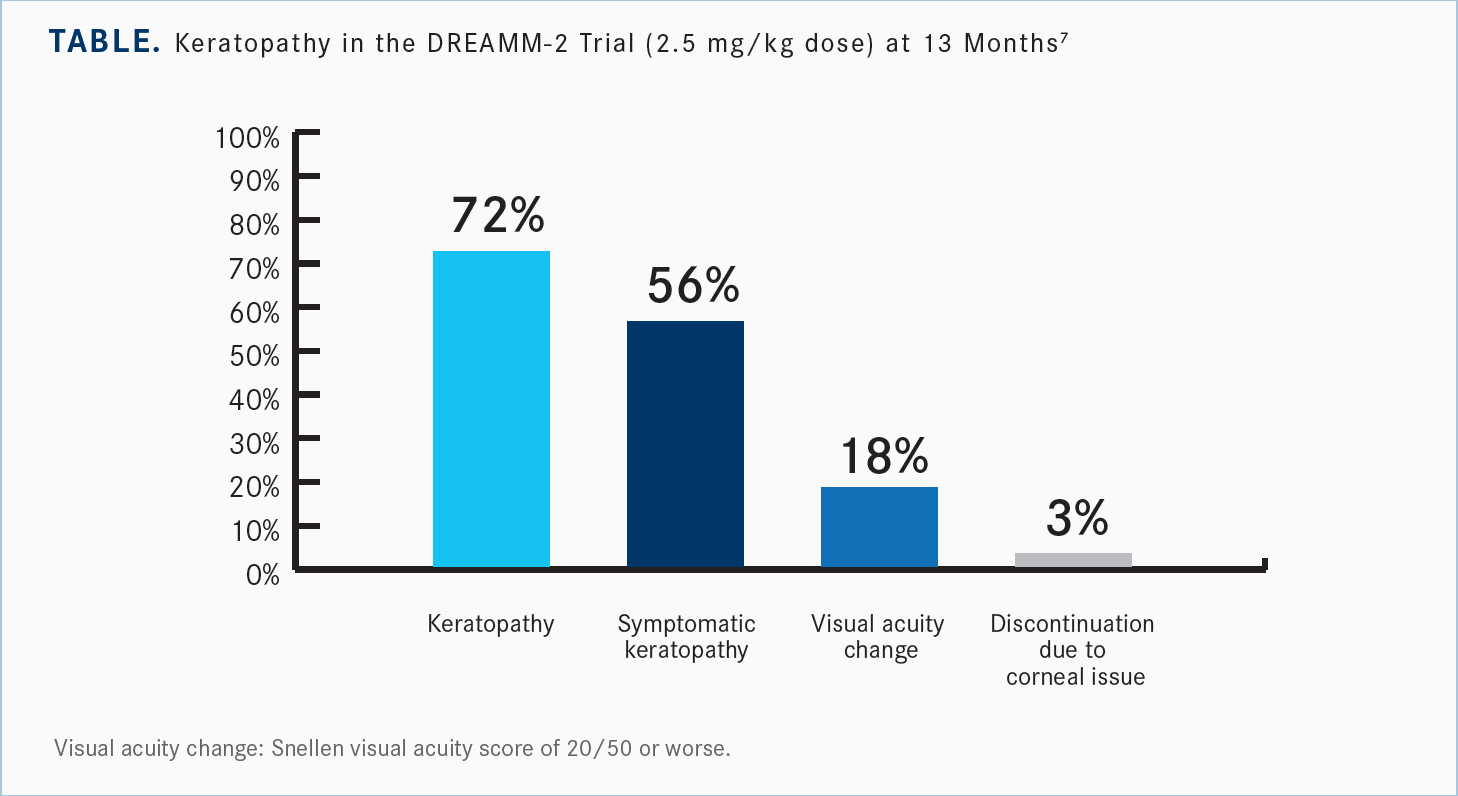
The ocular toxicities are sort of a spectrum. Even though the majority of these patients have some ocular toxicity, only approximately 3% have to permanently discontinue just because of the corneal adverse events.5 Even after 13 months of follow-up, [investigators] didn’t see any new safety signals with belantamab [Table7]. There was 1 patient with a grade 4 corneal ulcer, which obviously is very difficult and painful and more difficult to heal. But 82% of these patients did not have any significant clinical visual acuity change; a lot of these were just pitting of the cornea or subclinical findings that don’t necessarily have to make the patient stop. Certainly, if their visual acuity is affected, then we’ll want to make some changes.
Just remind everyone before using belantamab [that patients should] have a baseline eye exam within 3 weeks of the first dose, then subsequent ones at least 1 week after a dose but within 2 weeks prior to the next dose.6 [You should] withhold it if they’re having ocular toxicity, until improvement. It depends on what doses they’ve had before and how many doses before when you decide if you want to reduce it or delay it. We do have these patients using preservative-free lubricant eye drops 4 times a day starting right before they start these infusions and continuing throughout to prevent ocular toxicity. One of the main things that we can do if they develop ocular symptoms is have them bump up the frequency of those drops. [They should] avoid contact lenses that could irritate the cornea and avoid driving if they’re having visual acuity changes.
There’s a grading system with grades 1, 2, 3, and 4, but it’s just levels of corneal toxicity and levels of decreased visual acuity.8 Often with grade 1 we could continue with the dose, but at grade 2 we’re going to hold and delay. It depends on how severe it is if we want to reduce that dose.
What do the clinical data suggest about this patient?
Our patient had some blurry vision, keratopathy, grades 1 and 2—different on each eye—and we increased the frequency of the lubricant eye drops. He had a delay of a month for his second dose, until improvement to grade 1 or better. In this case, he resumed at the same dose. Now, I would argue that if you’d had 2 doses and then developed it, it might make sense to just delay it, then have an increased interval and use the same dose. But if [a patient] developed it after 1 dose, it’s probably going to happen again. So if it were me, I’d probably reduce the dose at that point. But he did attain a VGPR after the second dose. He was continued on therapy. He had further interruptions for grades 2 or 3 corneal events, with blurred vision, dry eyes, and photophobia. Then he had a dose reduction.
I just want to point out there are some data, including 5 patients [with 2 prolonged dose delays (PDDs)], where there were more extensive data showing that even if some of these patients have PDDs, they can still attain benefit from this therapy.7 Even if we have to delay it for quite a while, some of these patients can still have ongoing remission. It’s got a pretty long half-life, and sometimes you don’t need it more than every 3 to 6 months in some patients, if they’re having difficulties staying on it more frequently. And as I mentioned, I typically would start out at every 4 weeks, unless they’re progressing rapidly, and then extend the duration if they’re having trouble.
There are a few different quality-of-life [QOL] measurements that were done in the DREAMM-2 study.9 All 3 of these [QOL measurements] had either stable to slightly improved QOL, or at least didn’t have any significant worsening despite ocular events. There was generally improvement in fatigue, probably because they were off all their steroids and PIs and all the other therapies that they’d been on for 10 years before going on this trial.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 5.2022. Accessed June 24, 2021. https://bit.ly/3JZWq3p
2. Dimopoulos MA, Moreau P, Terpos E, et al; EHA Guidelines Committee. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):309-322. doi:10.1016/j.annonc.2020.11.014
3. Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e105-e118. doi:10.1016/S1470-2045(20)30756-7
4. FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. FDA. Updated August 6, 2020. Accessed June 24, 2022. https://bit.ly/3ODnQOL
5. Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221. doi:10.1016/S1470-2045(19)30788-0
6. Blenrep. Prescribing information. GlaxoSmithKline; 2020. June 24, 2022. https://bit.ly/3yi5IVr
7. Lonial S, Lee HC, Badros A, et al. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer. 2021;127(22):4198-4212. doi:10.1002/cncr.33809
8. Risk Evaluation and Mitigation Strategy (REMS) document: Blenrep (belantamab mafodotin) REMS program. FDA. August 5, 2020. Accessed June 24, 2022. https://bit.ly/3GWBpFD
9. Popat R, Lonial S, Voorhees PM, et al. DREAMM-2: single-agent belantamab mafodotin (Belamaf) effects on patient-reported outcome (PRO) measures in patients with relapsed/refractory multiple myeloma (RRMM). Abstract presented at American Society of Hematology Annual Meeting and Exposition; December 6, 2020; virtual. Abstract 2278. https://bit.ly/39TTHfh












