Powell Reviews Trials of PARP Inhibition in BRCA-Positive Ovarian Cancer
During a Targeted Oncology case-based roundtable event, Matthew Powell, MD, discussed trials supporting the use of the PARP inhibitors niraparib and olaparib for patients with BRCA-positive ovarian cancer.
Matthew Powell, MD
Professor, Obstetrics and Gynecology
Chief, Division of Gynecologic Oncology
Washington University School of Medicine
St Louis, MO

Targeted OncologyTM: What are the key trials of first-line maintenance therapy for patients with ovarian cancer following platinum-based chemotherapy?
POWELL: The primary trials have been established over the last 10-plus years, with the first being GOG-0218 [NCT00262847], which was a very large study looking at the role of the addition of bevacizumab [Avastin].1,2 And then [there were trials] looking at the PARP inhibitor experience with SOLO-1 [NCT01844986] and PRIMA [NCT02655016].3,4 And then is the PAOLA-1 trial [NCT02477644] looking at the combination of bevacizumab and olaparib [Lynparza].5 All these trials are up-front therapy studies followed by maintenance.1-5 The bevacizumab was also given concurrently with the chemotherapy.1
Most of our patients with ovarian cancer, unfortunately, present with advanced-stage disease. And that was what is represented in these trials; 70% to 80% of the patients had stage III disease, and a somewhat smaller proportion had stage IV.1-5 As we try to balance interpretations of some of these studies, we want to understand how much disease was left after their primary surgery and whether the patient had no [prior] surgery, such as in the PAOLA-1 trial. As we have learned, there are certainly indicators of response to not only chemotherapy but also to PARP inhibition, [through] understanding the patient population and whether there is germline or somatic BRCA gene mutations or HRD [homologous recombination deficiency]. These are some features that we look at.
Focusing on the January version of the NCCN [National Comprehensive Cancer Network] guidelines, it’s fairly generic through stage II to IV disease.6 Those patients are getting a platinum compound, best supportive care, and symptom management after therapy, and certainly thinking about maintenance therapy is indicated. When we look at up-front regimens, the most commonly used regimen is probably carboplatin/paclitaxel every 3 weeks, which has been the standard for many years. Whether you add bevacizumab, as in ICON-7 [ISRCTN91273375] or GOG-0218, is still a bit of debate.1,2,6,7 And other regimens that physicians would utilize: maybe substituting a taxane or even liposomal doxorubicin up front for patients [experiencing] unusual toxicities.6 Currently intraperitoneal chemotherapy is still out there, although it has been used less and less with some of the more recent trials. For the patients with carcinosarcoma, certainly the option of ifosfamide-based [Ifex] regimens are there, but hardly anybody utilizes these. I just finished a large, randomized trial that included carcinosarcomas, and I would say there is very little enthusiasm for using ifosfamide-based regimens for these patients.
When we look at what to do after therapy, a lot of this is determined by what we decide to do with their initial therapy. That initial decision tree is in the use of bevacizumab or not, and also whether or not the patient is known to have a BRCA gene mutation, either a germline or a somatic mutation. For a patient with BRCA wild-type or unknown disease, a complete or partial response we can observe, use niraparib, or use additional options. Similarly, if they have a germline mutation, we have other options or use of olaparib, niraparib, or observation, especially for stage II disease.6 If we use bevacizumab in the up-front setting, there is a similar decision tree, only we can use the homologous recombination proficiency [HRP] or HRD to determine which patients would receive a combination of olaparib plus bevacizumab, or bevacizumab alone. There are many different options for outpatients, and we hope to make a little bit of sense of what are the best options that we should be thinking about.
What data support the use of olaparib maintenance for patients with ovarian cancer?
Focusing on SOLO-1, when we think about major advances in gynecologic malignancies, I think if we had to say in the last decade, most physicians would put this up here near the top. In SOLO-1, all patients had either germline or somatic BRCA mutations, and it was almost all germline mutations with newly diagnosed, high-risk ovarian cancer, and patients had finished their platinum-based chemotherapy.3 Then they were randomized in a 2:1 fashion to olaparib vs placebo, and the primary end point was an investigator-assessed PFS [progression-free survival]. There was also a look at second PFS [PFS2], OS [overall survival], and health-related quality of life. And most of these patients had no evidence of disease at the end of their chemotherapy and had good performance status. Again, a lot of these BRCA patients were typically younger and healthier, and most of them had a normal CA-125 when they went on the study.
In the primary analysis, there was a hazard ratio [HR] of 0.3 [favoring olaparib; 95% CI, 0.23-0.41; P < .001]. At a median of 5 years of follow-up, [there is] the HR of 0.33 and quite a staggering difference [of a median PFS of 56 months with olaparib vs 13.8 months with placebo (95% CI, 0.25- 0.43)].8 The median treatment duration was a little over 24 months for the patients on olaparib, and a little less than 14 months on placebo.
We saw a lot of patients coming off treatment within a year. Almost half the patients are usually coming off due to secondary progression within a year vs almost 90% of patients still on therapy, and, again, this is 2 years of therapy, so that the treatment was capped at 2 years. And there were quite striking differences. The OS data are still not yet mature. We are looking at probably the next year or 2 before we will see the OS. And again, patients could cross over while this trial was going on. But this is one of the biggest differences when you think about outcomes in ovarian cancer; anything with an HR of nearly 70% risk reduction is pretty impressive.
Looking across the subgroups with PFS with 41 months of follow-up, every subgroup seemed to have benefit of the maintenance approach of olaparib, and some have made more of this BRCA2 group vs BRCA1, but, obviously, there were smaller numbers in the BRCA2 group.3 But certainly all seemed to benefit, and there were no outlier groups. When we look at the AEs [adverse events] with a greater than15% rate, [the safety profile remained consistent at the 5-year follow-up].3,8
No additional cases of MDS [myelodysplastic syndrome] or AML [acute myeloid leukemia] was reported, and the incidence of additional new malignancies were balanced between the 2 arms. Most of what we are seeing was in line with the initial experience: fatigue, nausea, vomiting, minor diarrhea. And most of this was low-grade, but within the grade 3 to 4 severity, the anemia was certainly a feature; that is probably the most noticeable for patients [who received olaparib] vs within the placebo population. I think when SOLO-1 first came out, many practitioners were rather [inexperienced] in the use of PARP inhibitors, but I think many of us feel comfortable in the use of these medications, and patients are much more accepting as well, as they have heard much more about it and feel comfortable with this AE profile.
What were the outcomes of the PRIMA trial of niraparib (Zejula) for patients with high-risk ovarian cancer?
When we focus on PRIMA, which is niraparib vs placebo, this is a little patient population with higher-risk ovarian cancer, and they could have any type of BRCA status: BRCA wild type, BRCA unknown, and BRCA mutated.4 [This trial was a] double-blind, randomized, placebo-controlled trial that was expanded from [the population in] SOLO-1 to include these patients with BRCA wild type, and it had not only just high-grade serous disease but also high-grade endometrioid. Within this we were getting a little smarter when this trial was designed, and also looking at the role of HRD and utilizing the Myriad myChoice tests to report that.
There are a few different tests out there, but this was what was embedded in this trial and probably has the most prospective data. The patients received a starting dose of either 200 mg or 300 mg of niraparib based on their body weight or platelet count, or a fixed dose starting at 300 mg. Throughout the trial [the investigators] started to figure out which patients were most susceptible to thrombocytopenia and were able to predict that using a weight and pretreatment platelet count to help dose the lower amount. The trial was a 2:1 randomization, niraparib starting at either 300 mg or 200 mg once daily vs placebo. The stratification factors were whether they had prior neoadjuvant chemotherapy with a complete response or partial response, and then their HRD status, whether it was positive, negative, or not determined.
When we look at the patient characteristics of this trial, again we looked at the fixed-dose population, which was 481 patients, vs 247 patients who had individualized dosing—and again, this happened partway through the trial.4 There was a fairly similar group as far as the stage, a fairly equal amount who were stage IV and who had prior neoadjuvant chemotherapy. When you look at this compared with the SOLO-1 population, a lot more patients were getting neoadjuvant chemotherapy, implying higher-risk disease. This was mostly a population with serous disease and a minor component of patients with endometrioid carcinoma.
When we look at the overall population, [there was a median PFS of 13.8 months in the niraparib group vs 8.2 months in the placebo group, with] an HR of 0.62 [95% CI, 0.50-0.76; P < .001], but again this was a little bit higher-risk population [than SOLO-1]. And we looked specifically at that HRD population, which has a median PFS of 21.9 months in those treated with niraparib vs 10.4 months with placebo with an] HR of 0.43, nearly 12 months’ increase in median PFS, a fairly striking difference [95% CI, 0.31-0.59; P < .001 (Table4)]. Twenty percent of patients progressed in the first few months. You can look at these Kaplan-Meier curve morphologies and see that you have a high-risk population that has benefited. It appears to have good benefit with the use of a maintenance PARP inhibitor. These patients stayed on therapy for up to 3 years.
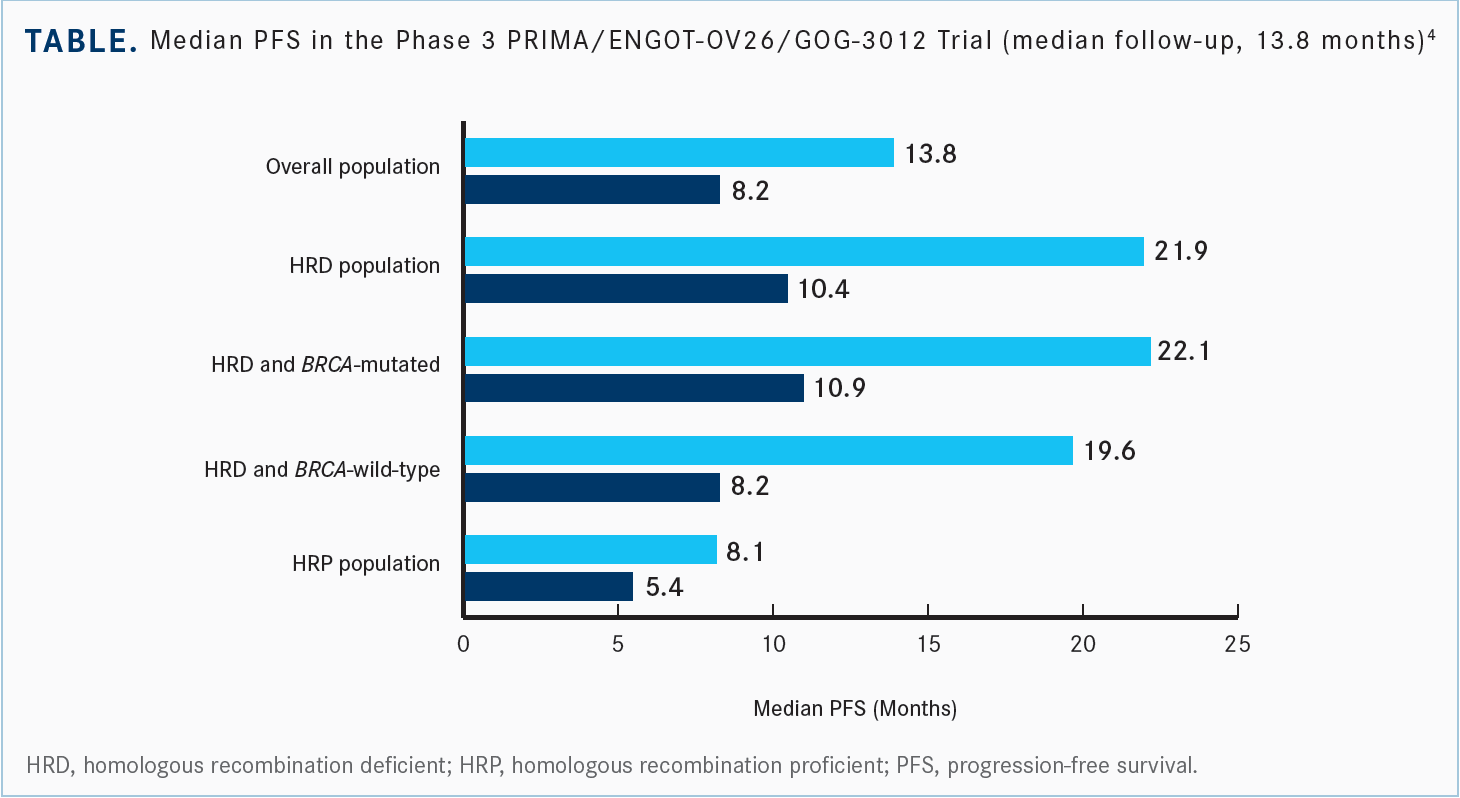
When we look at the specific populations, the difference between niraparib and placebo in HRD/BRCA-mutated patients was an HR of 0.4 [95% CI, 0.27-0.62] and an HR of 0.5 in HRD/BRCA wild-type [95% CI, 0.31-0.83]. These are fairly similar results, whether their HRD status was due to BRCA mutation or not.4 Looking across at the different subgroups, there were no outliers there. It is between stage III and IV, whether they had neoadjuvant chemotherapy, and no special groups of interest other than the small cohort of patients where they were not able to determine the HRD status, but again, there was a wide range [for their PFS]. But all the median PFS estimates are favoring the use of niraparib.
Interim analysis of OS certainly favored niraparib over placebo.9 Again, this was quite premature. The OS event rates were low, while 84% of patients who received niraparib vs 77% of patients who received placebo were alive at 2 years. And then if the patient had HRD, 91% vs 85%, respectively, were still alive. And for those who were HRP: 81% vs 59%, respectively.
So, this was somewhat intriguing to see this HRP population having this difference. Again, not sure what to make of that, given the low numbers, but we certainly see the patients with HRP do worse. Still waiting to see these [data when they are] a little bit more mature and see if this difference holds up for the patients. We thought maybe PARP inhibitors aren’t doing as much for HRP patients, but this little signal in OS is intriguing. Some physicians have [enrolled certain patients because] this is their 1 opportunity to get a PARP inhibitor, and that is helping to affect the OS. But, again, it is a bit premature to state that. Looking at safety, anemia, nausea, and then thrombocytopenia were [the most common AEs] of the trial.4 This was before use of the dosing based on the patient weight or their pretreatment platelet count. Certainly thrombocytopenia in the treatment group was more of an issue when you look at the rate of AEs of grade 3 or higher, of about 29% of thrombocytopenia, 31% of anemia.
There was 1 patient diagnosed with myelodysplastic syndrome at 9 months on niraparib treatment, but no new safety signals were identified, and most of these treatment-emergent AEs were reversable on myelosuppression. With dose reductions and dose delays, most of this goes away. We do see MDS, but thankfully, this is not as bad a problem as when these drugs were first coming out, when we thought it would be much more of an issue.
What were the findings of the PAOLO-1 trial of the combination of olaparib and bevacizumab?
The PAOLA-1 trial of platinum-based chemotherapy plus bevacizumab vs olaparib plus bevacizumab maintenance was an investigator-initiated trial run in Europe.5 Patients had newly diagnosed advanced ovarian cancer and a good performance status. They had either partial or complete response; it was 6 to 9 cycles of therapy, and neoadjuvant chemotherapy was allowed. Patients had no prior PARP inhibitor exposure, and these patients received bevacizumab vs bevacizumab plus olaparib or placebo.
It was quite intriguing. Before this study came out, many would have thought, “How much is a PARP inhibitor going to add when the patient is already getting the benefit of bevacizumab?” And it was quite striking. In the prespecified subgroup analyses, patients with tumor BRCA mutations and positive HRD status had the greatest PFS benefits.5 We don’t have OS data yet. But, in looking at the PFS data with a HR of 0.59, we are seeing some quite impressive median PFS differences, with 18-month PFS rate of 62% with olaparib plus bevacizumab vs 46% when receiving the placebo. And at 24 months, almost twice the PFS rate [with olaparib plus bevacizumab at 46% vs 28% with placebo plus bevacizumab].
If we look at patients who are HRD positive, including those with tumor BRCA mutations, they had an HR of 0.33, while those who were HRD positive excluding the tumor BRCA mutations had an HR of 0.43, and then those who were HRD negative or unknown had an HR of 0.92.5 Some have said this population may [have patients] who were paying close attention, and realized that HRD-negative was not part of the guidelines for this combination regimen of olaparib and bevacizumab. In the biomarker subgroups, most of the patients did benefit when they either had a BRCA mutation or an HRD that was negative or negative unknown or unknown. The addition of the olaparib to the bevacizumab did not seem to matter. When we look at AEs occurring at 10% or greater, again, you can certainly [apply] most of the PARP experience, and then you can add hypertension with that, given the addition of bevacizumab.5
There was nothing that was unexpected here. Fatigue was certainly greater with the addition of olaparib, but overall quite manageable. I think at this point now, several years into the use of PARP inhibitors, we have learned that with dose delays and [short treatment] holidays and such, we have done well to find the appropriate dose for these patients to keep them on therapy and manage the fatigue factor and occasional nausea.
Which AEs are the most challenging when using a PARP inhibitor in the primary maintenance setting?
When we look at selected toxicities, anemia, thrombocytopenia, neutropenia, and when we look at all grades and then grade 3 or 4 AEs, the platelet toxicity is a little more on the niraparib compound, but when we can maybe start the 200-mg dose, it is a little bit less. Once patients are on this for a time, it is not a cumulative situation—these platelet counts usually drop rather quickly. But once you get the patients on the right dose, that is not as much of a problem.
The nausea certainly can be noted. The amount of grade 3 or 4 nausea is relatively small, but chronic nausea can be a problem, and we will talk about some of the strategies for that. Sometimes giving a nighttime dose of the medication, where they have their nausea at night, is nice with the single-day dosing of niraparib. Twice-daily dosing with olaparib may not be quite as easy. We are using the olanzapine [Zyprexa] compound; it’s been nice for the nausea/vomiting, although most of it is lower grade, and most of it goes away with time, and it is usually a feature of the initiation of therapy. Hypertension is mostly with the bevacizumab-containing compound. But we do see it with…niraparib, where we will see minor elevations in blood pressure as well.
How can physicians use the individualized dosing approach for niraparib, and how should they use dose adjustments for PARP inhibitors?
For individualized dosing [of niraparib] in a primary maintenance setting, it was found that those patients who were under 77 kg or with a platelet count of under 150,000 were most at risk for that increased platelet toxicity early in their use of niraparib. So patients weighing greater than 77 kg and with at least platelet counts of 150,000 [should] start at 300 mg. [This is] an either-or situation, so if patients are either a lower weight or they have a low platelet count, we can start those patients at 200 mg. And similar outcomes have been noted for the PFS with patients with an individualized starting dose in the exploratory analysis. So that nice HR [favoring niraparib] was maintained.
If we consider the dose adjustments for AEs, olaparib initially was available in capsules and tablets [before capsules were discontinued], so some of this was a little confusing initially. But the starting dose for olaparib is 300 mg twice daily, and that’s 2 of the 150-mg tablets per dose, and your first reduction is down to 250 mg, and secondary reduction down to 200 mg.10 With niraparib, they came in 100-mg capsules.11 Again, if patients had both a weight over 77 kg and good platelet count, that’s a 300-mg dose, [first] reducing to 200 mg, then reducing to 100 mg, or [for lower weight or platelet count], then starting at 200 mg, [first] reducing to 100 mg [and then discontinuing]. So, it’s pretty straightforward from that standpoint. Again, this is once-a-day dosing with niraparib and twice-daily dosing with olaparib.9,10
What additional data are there to support the individualized dosing approach for niraparib?
The PRIME study was a follow-up study done primarily in Asia, looking at this individualized starting dose [of niraparib]. These patients were randomized to 2:1, niraparib vs placebo. They were on for 36 months until disease progression, and so the starting dose if you were under 77 kg was 200 mg, basically following that weight and platelet designation, and this was done prospectively throughout the whole trial rather than partway through as in the PRIMA study.
Again, with individualized dosing we still see very nice results, with the niraparib vs placebo showing an HR of 0.45 [favoring niraparib], and treatment was tolerated with an improved safety profile, and the treatment-emergent AEs that led to discontinuation were quite manageable at 6.7%.11 [There is] not much else to say other than throughout the different groups the HR was maintained, whether they had HRD, germline, or nongermline mutations. The proportion of patients that were without progression or death at 24 months was 52% with niraparib vs 30% with placebo. These were nice results, consistent with the results from the prior study that individualized dosing seems to make sense as the way to mitigate toxicity, especially that platelet toxicity in patients who are going to be treated with niraparib.
Safety is comparing the PRIME study, where the whole study was individualized dosing, vs PRIMA for the different grades of toxicity.4,12 And the key was that treatment-related AEs leading to dose reduction was about 40% for those receiving niraparib in PRIME vs 70% in PRIMA. But you could say that a large portion of patients on PRIME [effectively] had a dose reduction at the very beginning, balancing that out. But [it was] consistent, and there were no new safety signals seen in the individualized-dose group. So, we avoid some of that worrisome thrombocytopenia by dosing the patients based on their platelet count and their weight.
REFERENCES
1. Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473- 2483. doi:10.1056/NEJMoa1104390
2. Norquist BM, Brady MF, Harrell MI, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: an NRG Oncology/Gynecologic Oncology Group study. Clin Cancer Res. 2018;24(4):777-783. doi:10.1158/1078-0432.CCR-17-1327
3. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495- 2505. doi:10.1056/NEJMoa1810858
4. González-Martín A, Pothuri B, Vergote I, et al; PRIMA/ENGOT-OV26/ GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi:10.1056/ NEJMoa1910962
5. Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416-2428. doi:10.1056/NEJMoa1911361
6. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer/fallopian tube cancer/primary peritoneal cancer, version 1.2022. Accessed June 29, 2022. https://bit.ly/2Nr8vCu
7. Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928- 936. doi:10.1016/S1470-2045(15)00086-8
8. Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1721-1731. doi:10.1016/ S1470-2045(21)00531-3
9. Lynparza. Prescribing information. AstraZeneca Pharmaceuticals; 2022. Accessed June 29, 2022. https://bit.ly/3bB4Hii
10. Zejula. Prescribing information. GlaxoSmithKline; 2022. Accessed June 29, 2022. https://bit.ly/3Aojuae
11. Li N, Zhu J, Yin R, et al. Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): a randomized, double-blind, placebo-controlled, phase 3 trial. Presented at: 2022 SGO Annual Meeting on Women’s Cancer; March 18-21, 2022; Phoenix, AZ
12. Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi:10.1056/NEJMoa1611310

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More













