Porter Reviews Chemoimmunotherapy Combination Data in NSCLC
During a Targeted Oncology™ Case-Based Roundtable™ event, Jason Porter, MD, discussed the CheckMate 9LA and KEYNOTE-407 trials of chemotherapy combined with immunotherapy in patients with advanced squamous cell lung cancer.
Jason Porter, MD
Director, Lung Cancer Disease Research Group
West Cancer Center and Research Institute
Memphis, TN

Targeted Oncology™: What are the key trials that explored combination immunotherapy and chemotherapy in squamous cell non–small cell lung cancer (NSCLC)?
PORTER: CheckMate 9LA [NCT03215706] used 2 cycles of chemotherapy plus nivolumab and ipilimumab vs 4 cycles of platinum doublet.1 And in KEYNOTE-407 [NCT02775435], it’s pembrolizumab [Keytruda] plus carboplatin plus paclitaxel [Taxol] or nab-paclitaxel [Abraxane].2 These are positive first-line advanced NSCLC trials.
Can you discuss the details of the CheckMate 9LA trial?
CheckMate 9LA is a relatively large trial that had nivolumab plus ipilimumab plus chemotherapy vs 4 cycles of platinum-doublet chemotherapy.1 It included 361 patients randomly assigned to the intervention arm vs 358 patients in the chemotherapy arm. They were eligible if they had stage IV or recurrent NSCLC, no prior therapy, no ALK or EGFR alterations, and good performance status. They were stratified by PD-L1 level of less than 1% or greater than or equal to 1%, sex, and squamous vs nonsquamous histology. They were treated until disease progression, unacceptable toxicity, or 2 years with immunotherapy.
The primary end point was overall survival [OS]; secondary end points were progression-free survival [PFS], objective response rate [ORR], and efficacy as it related to the PD-L1 expression level. And [the trial had] an exploratory end point of safety. At 3 years of follow-up, it was exciting to see that of the patients treated with nivolumab-ipilimumab plus chemotherapy, 27% of them were still alive vs 19% of the patients treated with chemotherapy alone.3
This is a statistically significant difference. The median OS was 15.8 months vs 11.0 months, with an HR of 0.74 [95% CI, 0.62-0.87]. There was a 26% reduction in death at 3 years for patients treated with nivolumab-ipilimumab with just 2 cycles of platinum-doublet chemotherapy. If we look at the PFS, as well as the duration of response [DOR] and ORR, the median PFS was 6.4 months vs 5.3 months [for chemotherapy alone], with an HR of 0.70 [95% CI, 0.59-0.83].3
Then the ORR was 38% in the nivolumab-ipilimumab-chemotherapy group vs a 25% ORR in the chemotherapy group. The median DOR was more than double with the nivolumab-ipilimumab-chemotherapy, at 12.4 months vs 5.6 months. And [there was] an [ongoing] response at 3 years in nearly a quarter of the patients treated with nivolumab-ipilimumab-chemotherapy, at 23% vs 14% in the chemotherapy-alone group.
We also looked at the 3-year OS, as broken down by PD-L1 level.3 For patients with PD-L1 greater than or equal to 1%, 28% of those patients [who received ipilimumab/nivolumab] were alive at 3 years [vs 19% with chemotherapy alone]. And PD-L1–negative OS at 3 years was 25% vs 15% with the chemotherapy alone—so not much difference in the PD-L1–expressing group vs the nonexpressers. For responding patients with PD-L1 of less than 1% in the Checkmate 9LA trial, is it possible that ipilimumab is the more active agent, since nivolumab is the drug that inhibits PD-L1?
That’s probably a multifaceted difference. When we see “PD-L1 negative,” we have to look at how effective the PD-L1 staining was. A second issue is tumor heterogeneity: If we biopsy a sample site and find that PD-L1 is not expressed there, does it mean that they’re not expressing? Or is there going to be a spot just a few millimeters to the left or right—or even centimeters to the left or right— that’s actually expressing PD-L1?
So I’d caution [against] believing that it’s the ipilimumab working vs the nivolumab. Maybe PD-L1 expression was erroneously negative, or there was tumor heterogeneity. I would lean more toward that as the explanation, given that I don’t believe that there’s much difference between nivolumab and pembrolizumab. With ipilimumab targeting CTLA-4, I think there may be a difference there.
In the PD-L1–high expressers of greater than or equal to 50%, there was a 33% OS rate at 3 years for those patients [who received chemoimmunotherapy, vs 24% with chemotherapy].3 In a stepwise fashion, the higher the PD-L1, the higher the OS at 3 years, [which is] what we expect to see. But I do agree that nivolumab-ipilimumab alone has some survival benefit even for those patients without PD-L1 expression. Again, I think biomarker selection is going to be important. The more we learn about these, the better we’ll be able to predict response to therapy.
Looking at the treatment-related adverse events with patients treated with nivolumab-ipilimumab-chemotherapy, most of them are grade 1 or 2, and there is a sliver of grade 3/4 at the top of each of those bars.1 Toxicity: mostly pruritus. But sometimes we’ll get rash, and of course, grade 3/4 will be a little bit more severe, covering more body surface area. The endocrine adverse events [AEs]—hypothyroidism, adrenal dysfunction, and pancreatic dysfunction—don’t happen often. Thyroid toxicity, of course, is very common though. If we look at the GI [gastrointestinal] toxicity, it’s mostly grade 1/2, but there’s a little bit more grade 3/4, so maybe diarrhea and colitis. [There were also] hepatic, renal, pulmonary, and hypersensitivity/infusion reactions.
What does this trial show about the tolerability of immunotherapy?
We’ve become comfortable using these for monotherapy or in combination. And there has been much discussion over the past few years about the dose that we use in NSCLC and the difference from melanoma dosing. So even with the combination, I think most of us are pretty comfortable using [it]. I don’t see much increased toxicity. Every now and then I’ll see a little increased arthritic reaction in my patients on the cycle [when] they get the nivolumab and the ipilimumab, but usually that’s pretty easy to [manage].
When we look at the AEs typically associated with chemotherapy [that were reported in this trial], these are mostly cytopenias, neuropathy, and alopecia—typical chemotherapy AEs that we’re all used to managing.1,4 Most of them are grade 1/2, but then we do see some grade 3/4 anemia and neutropenia, as well as febrile neutropenia and thrombocytopenia. I do believe from the trial there was even a patient who had catastrophic thrombocytopenia with a fatal hemorrhage. So we have to watch out for the toxicity: the bone marrow toxicity, then alopecia. But it’s mostly well tolerated, and there’s not much difference between chemotherapy and immunotherapy.
[Looking at] the efficacy as it relates to patients who had to discontinue their therapy due to immune-mediated AEs or treatment-related AEs, the median OS of these patients was 27.5 months; 54% of them were alive at 2 years vs 38% in the chemotherapy arm [Figure4]. The ORR was 31%. And we see the ongoing response at greater than or equal to 1 year after discontinuation: 56% of those patients still responding, even a year after discontinuing therapy for immune-mediated AEs.
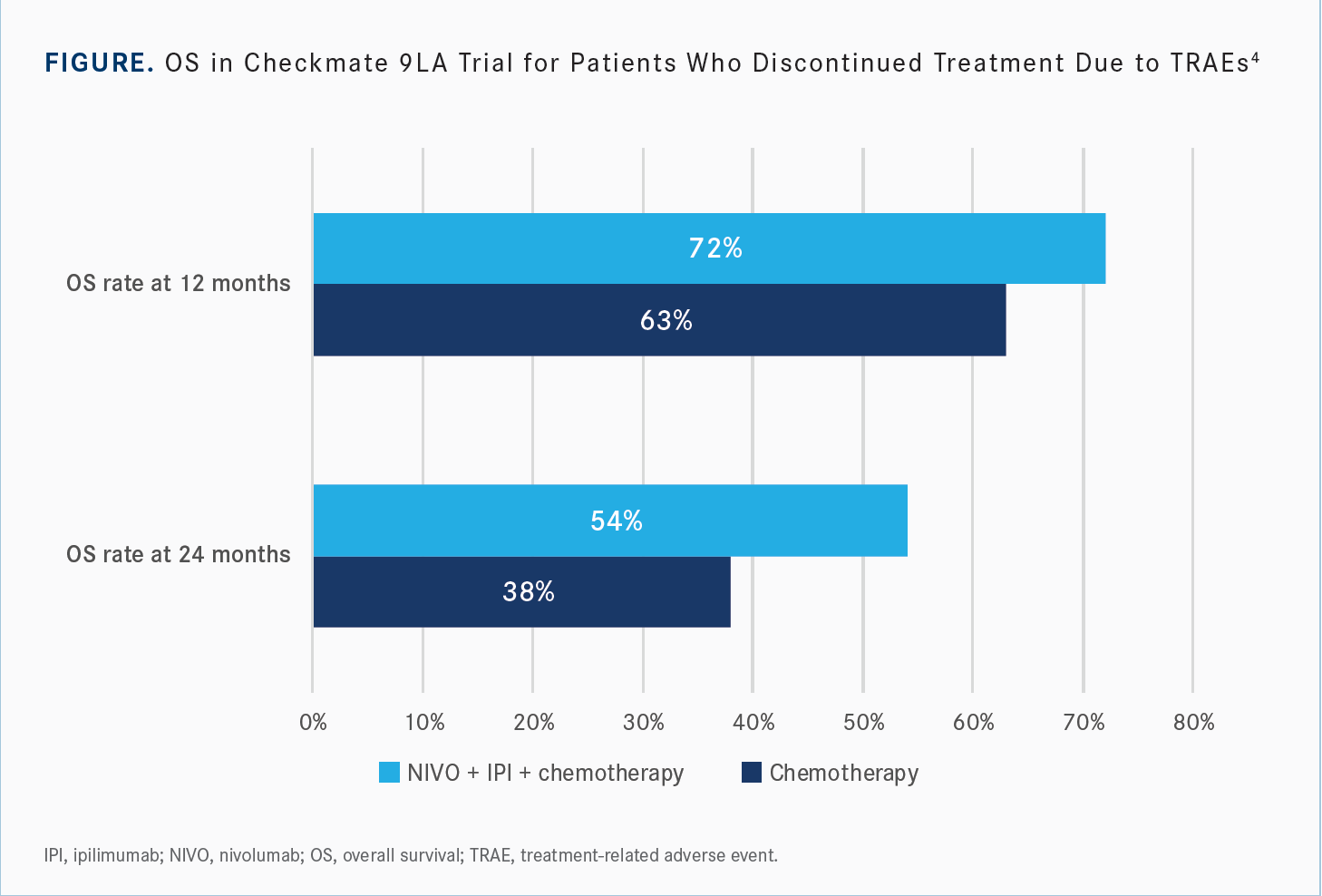
It’s kind of intuitive when you think about it. The patients who have immune-mediated AEs are likely those having a robust immune response, and those are the ones we see have disease control. Unfortunately, sometimes that’s a trade-off for quality of life, depending on which one of those immune-mediated AEs patients experience. But, if they do, we expect to see disease control even up to a year after those patients have stopped therapy.
Could you discuss the results of the KEYNOTE-407 trial?
In the KEYNOTE-407 trial, patients were randomly assigned on a 1:1 basis to pembrolizumab plus carboplatin AUC [area under the curve] 6 mg/mL/min plus paclitaxel or nab-paclitaxel for 4 cycles, followed by pembrolizumab maintenance for up to 31 cycles in one arm. And in the placebo arm, they received placebo plus carboplatin plus paclitaxel or nab-paclitaxel, followed by placebo [maintenance].2 Then the placebo-arm patients had an optional crossover at the time of progressive disease.
These patients all had untreated stage IV squamous cell histology, good performance status [0 or 1], provisions for a PD-L1 assessment sample so they had to have tissue available for PD-L1 assessment, no symptomatic brain metastases, and no pneumonitis requiring systemic steroids. They were stratified by PD-L1 expression of less than 1% or greater than or equal to 1%; the choice of taxane, paclitaxel vs nab-paclitaxel; and then geographic region, East Asia vs the rest of the world. PFS and OS were coprimary end points, and then secondary end points were the ORR and DOR, as well as safety.
There was a 15.9-month median OS [with the pembrolizumab combination] vs an 11.3-month median OS [with the placebo combination].2 In the pembrolizumab-plus-chemotherapy group, about 31% of those patients had OS events vs 42% or 43% of the placebo-plus-chemotherapy group having events, with a 36% reduction in death from the cancer [for those who received pembrolizumab]—and it was statistically significant [HR, 0.64; 95% CI, 0.49-0.85; P = .0008]. For PFS, 54.7% of the patients in the pembrolizumab-chemotherapy group had progression events, and 70.1% in the placebo-chemotherapy group had progression events, with a 44% reduction in progression for those patients treated with the pembrolizumab plus chemotherapy [HR, 0.56; 95% CI, 0.45-0.70; P < .0001]. Median PFS was 6.4 months for pembrolizumab vs 4.8 months for placebo.
We see a slight difference in the taxane being used.2 For the patients who had nab-paclitaxel, there looked to be a slight advantage. For OS in that group, there was an HR of 0.59 [95% CI, 0.48-0.93] vs 0.67 in the paclitaxel group [95% CI, 0.36-0.98]. You have to look at the numbers there. Are the numbers equivalent? Does this make a difference? Are there any toxicity trade-offs for using one vs the other? If we select one and have a [slightly] better response, are we trading off for the toxicity? But, overall, most of the subgroups seemed to benefit from the addition of pembrolizumab in that combination.
Can you discuss the OS and PFS outcomes by PD-L1 expression subgroup in KEYNOTE-407?
[When] we look at the PD-L1 expression level and the OS in a stepwise fashion, up the ladder, we go from a PD-L1 tumor proportion score [TPS] of less than 1%, or negative, and then 1% to 49%, and then greater than or equal to 50%.2 We can see an increase in the OS and a meandering of the HR: 0.61, to 0.57, to 0.64 [in these respective subgroups]. The OS goes up, and the number of events goes down. [The rate of] events in the pembrolizumab-chemotherapy group at TPS less than 1% [is] 30.5%, [at TPS of 1% to 49% is] 30.1%, and then [at 50% or greater is] 31.5%.
It is the same thing with PFS.2 It’s a little bit more of a dramatic difference in the PFS. The median PFS in the TPS of 50% or greater group is 8.0 months, for the 1%-to- 49% group it is 7.2 months, and for the TPS of less-than-1% group it is 6.3 months. And all [subgroups treated with] pembrolizumab plus chemotherapy beat chemotherapy in those patients with squamous histology.
How was pembrolizumab plus chemotherapy tolerated vs chemotherapy plus placebo?
[In terms of] AEs…the grade 1/2 AEs occurring in more than 20% of patients, some of them were clearly more likely to be attributed to the chemotherapy, like anemia, alopecia, and neutropenia.5 Diarrhea could go either way, of course. Decreased appetite could go either way as well. And we do not see much difference between the 2 [arms]. Anorexia that goes along with immune checkpoint inhibitors should not be discounted though. We see anorexia in some of our patients, as well as fatigue, which is so classically associated with chemotherapy. But immune-therapy monotherapy, even in combinations without chemotherapy, can cause pretty significant fatigue. The arthralgias we see are increased in the patients given the immune checkpoint inhibitor plus chemotherapy vs the chemotherapy alone.
The immune-mediated AEs are obviously going to be at a higher frequency in the pembrolizumab plus chemotherapy group vs the chemotherapy group. Hypothyroidism [occurred at a rate of 7.9% in patients who received pembrolizumab].
[The next most common reported immune-mediated AE] was hyperthyroidism, probably with diarrhea and other symptoms of hyperthyroidism. [Next were] pneumonitis, colitis, hepatitis, [and] then skin reactions. I’ve had some patients with some pretty miserable skin reactions to pembrolizumab. I actually had to stop a patient’s therapy because he hadn’t slept in about 3 weeks when he came to see me because he was itching incessantly, and we had done everything. I’m at the point of asking dermatology about maybe trying apremilast [Otezla] for this patient because he’s still not reacting to steroids, to hydroxyzine, to gabapentin—everything I’ve tried. So these AEs can affect patients’ quality of life, and we can’t discount them. We have to monitor them really closely.
In patients with positive PD-L1, there is separation in the Kaplan-Meier curves for OS after 3 to 6 months, but in the PD-L1–negative group, there was no significant separation until around 7 months.2 Could this be explained by a change in PD-L1 expression later on?
I think that’s an interesting observation. There could be, and there is a belief that chemotherapy and radiation therapy can both change the PD-L1 expression. The other [possibility] is, are you selecting for, or selecting out, the cancer cells that are going to die [because of] the chemotherapy? Then we [would] see that effect up front, and the rest of what you see [would be] the pembrolizumab effect. When the PD-L1 is very high, that gives me a little bit of comfort [because of] the early separation in those curves.
For a very long time when I first started practicing, I would not give [immune checkpoint inhibitor] monotherapy; I was giving chemotherapy. But even in monotherapy, I’ve seen that when that PD-L1 is very high, there is usually a pretty rapid response to the immune checkpoint inhibitor.
There is [other] PD-L1 therapy that has a curve in its data that shows the decrease in tumor volume by PD-L1 level, and the higher the PD-L1 level, the more rapid the decrease in tumor volume. So it’s similar, but of course, here it is [combined] with chemotherapy. But when I see that rapid decrease in tumor volume at high PD-L1 levels, it makes me a little bit calmer and less anxious about giving monotherapy.
REFERENCES
1. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi:10.1016/ S1470-2045(20)30641-0
2. Paz-Ares L, Luft A, Vicente D, et al; KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi:10.1056/NEJMoa1810865
3. Paz-Ares LG, Ciuleanu TE, Cobo-Dols M, et al. First-line (1L) nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients (pts) with metastatic non-small cell lung cancer (NSCLC): 3-year update from CheckMate 9LA. J Clin Oncol. 2022;40(suppl 17):LBA9026. doi:10.1200/JCO.2022.40.17_suppl.LBA9026
4. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. J Clin Oncol. 2021;39(suppl 15):9000. doi:10.1200/JCO.2021.39.15_suppl.9000
5. Paz-Ares LG, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/ nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(suppl 15):105. doi:10.1200/JCO.2018.36.15_suppl.105
