Roundtable Discussion: Yu and Participants Assess the Role of PSMA Therapy for mCRPC
During a Targeted Oncology™ Case-Based Roundtable™ event, Evan Y. Yu, MD, discussed with participants role of prostate-specific membrane antigen in metastatic castration-resistant prostate cancer.

YU: This patient could be eligible for cabazitaxel [Jevtana] or [radium Ra 223 dichloride (radium 223; Xofigo)]. I think it is a bad idea to pick another AR-targeted therapy. But anyone want to tell me why they like lutetium Lu 177 vipivotide tetraxetan [177Lu-PSMA-617; Pluvicto] over cabazitaxel or radium Ra 223 dichloride?
GALAMAGA: I’ll say the efficacy is quite impressive, and I worry about adding more taxane to the patient in terms of affecting their performance status. I have a side question: How heterogeneous is prostate cancer? Can it express the PSMA, and then part of the disease process doesn’t [express it] and might be resistant? In the Phoenix, Arizona, I haven’t been able to access [lutetium Lu 177 vipivotide tetraxetan] yet.
YU: Prostate cancer is a heterogeneous cancer, but so far, I haven’t seen a ton of heterogeneity regarding PSMA expression yet. I imagine as time goes on and patients become resistant to this agent, we’ll see more and more heterogeneity of expression, and that’s natural [as] a marker of treatment resistance. But interestingly, most patients express it well.
Of course, there are some patients who have some dominant-negative lesions, and those are the patients who [were not selected] in the studies. So it’s hard to say how well they would do. The label still allows you to treat those patients,1 but we’re going to have to gather experience as time goes on as to whether it’s a good idea to treat those patients or not.
I think what’s happening right now is with limited access most patients don’t have other good options, so we treat them anyhow. But down the road, we might learn that maybe those patients don’t respond as well and that might be a patient you want to give cabazitaxel or radium Ra 223 dichloride to if they have predominantly bone metastases. WU: How do you define [PSMA] positivity?
YU: Just over liver uptake. Liver uptake [alone would be] very low [PSMA expression], but anything over liver uptake is considered positive.
WU: Is the [PSMA] expression ever correlated with response?
YU: Yes. There are 2 abstracts that have been presented. One was [from the] VISION trial [NCT03511664], which showed that it does [correlate].2 Here’s the problem: It’s impractical for us to do easily. Certainly [it would be impractical] in the community because they do total body SUV [standardized uptake value], and there’s apparently a lot of subjectivity. It’s not that objective to determine the whole-body SUV.
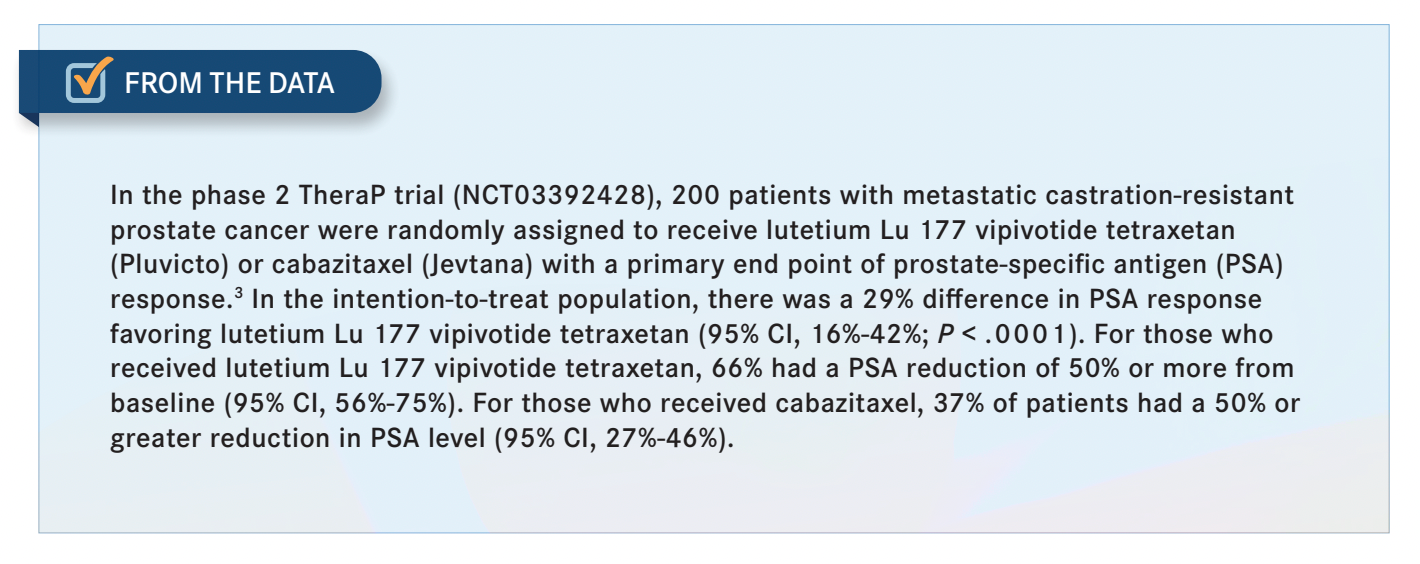
[The study investigators] summed it together, and then they were able to show that if you have higher-level uptake, you’re going to do better. I think it must be an SUV over 10. They did that both in the VISION trial and in the TheraP trial [NCT03392428], where they compared lutetium Lu 177 vipivotide tetraxetan with cabazitaxel.3 As time goes on, we might learn that it’s ideal for patients [with high uptake] to be treated with lutetium Lu 177 vipivotide tetraxetan and maybe patients with lower uptake to receive chemotherapy. So that’s still an evolving field, but that’s what it points toward now. It might not just be prognostic; it might have a predictive value there.
SALGANICK: Radium Ra 223 dichloride has a survival benefit versus placebo,4 so I just don’t know what to do with that drug. It seems like an expensive alternative, so that’s why I crossed that one off. I agree with you; I don’t like the ADTs. I would like to use something else [and not] cabazitaxel. We have a great solution, which I don’t know if it beat cabazitaxel, but it beat other [novel hormonal agents] and showed that it works, so why not? For the cost issues and access— only a few institutions have lutetium Lu 177 vipivotide tetraxetan here [in Phoenix], so I would use cabazitaxel.
YU: I think your rationale makes perfect sense. It beat the novel hormonal therapy agents in the VISION trial. In the TheraP trial, which was an Australian trial, it was superior to cabazitaxel based on the primary end point, which was a 50% or greater PSA decline [From the Data3 ]. The secondary end points, which were composite progression-free survival [PFS], radiographic PFS, and PSA-PFS, showed superiority over cabazitaxel and it was less toxic because cabazitaxel has more myelosuppression neuropathy, etc. Of course, you get more xerostomia or dry mouth with lutetium Lu 177 vipivotide tetraxetan, but overall, cabazitaxel had more fever, infections, all those sorts of [toxicities]. You’re right that it doesn’t have overall survival [OS] data; it wasn’t powered to show that, but if you look at other end points, it’s better than cabazitaxel.
SALGANICK: Lutetium [lutetium Lu 177 dotatate; Lutathera] caused renal failure in 2 [of my] patients and prolonged bone marrow suppression in 2 patients months after they finished it, but this was not in prostate cancer; it’s in carcinoid tumors.5 [Can I] borrow my experience from the carcinoid [setting], or is it a totally different [toxicity profile]?
YU: We see that as well in prostate cancer.1 One nice thing is it doesn’t cause as much myelosuppression as some of the other radiopharmaceuticals where they use an antibody. That’s an advantage to a small molecule, but the disadvantage is it’s smaller, so it gets into salivary glands and renal tubules that express PSMA. So you are going to see more renal tubular necrosis and renal dysfunction as a potential risk factor as well. The studies didn’t find a lot, but…once we start treating real-world patients, we’ll see more. The studies have more ideal patients.
SALGANICK: I have had 2 patients with renal failure. It’s pretty rough stuff.

VICUNA: For me, I think the toxicity profile is better with the lutetium Lu 177 vipivotide tetraxetan compared with cabazitaxel, so that would be one advantage in my opinion.
YU: So [your concerns are] quality of life and toxicity. That’s got to matter here.
AMBIKA: Do you know if the dry mouth is reversible? What are the management options for that, or is it permanent when they have it?
YU: It can be permanent if it’s bad enough. But certainly, if you get a little bit of therapy and stop, I’ve seen it improve in patients. There are methods [to] manage it. It’s a lot of education. I tell people to avoid alcohol and tobacco. Those things dry you out a lot more. Keep a humidifier in your room. Sip [water frequently]. Chew [gum containing xylitol]. Avoid mouthwashes. Those are all kinds of practical tips you can give to patients. There are artificial saliva substitutes, but…nobody likes using them.
KAHN: I’d have to echo what everyone else said. I’d love to see some better efficacy data. But for cabazitaxel, some patients don’t tolerate it very well, so is it bad to say if anything is better [for those patients], then it’s a plus?
YU: It’s not bad to say that. I think it’s fair. Where do you think it’d ultimately be used? Do you think this is going to be pigeonholed in the postchemotherapy space or maybe moved earlier and earlier and earlier? I’m not sure about going as early as [neoadjuvant therapy], but what about in the prechemotherapy mCRPC space? What about in new metastatic prostate cancer?
SALGANICK: I have a patient with new metastatic prostate cancer with uncertain lymph node [involvement] in the pelvis. I was going to just put him on chemotherapy because I didn’t know if this was hormone-sensitive [disease]. Then I brought him to a tumor board. They said you can use the piflufolastat F 18 [injection (Pylarify)] PSMA scan. It’s FDA approved for that exact purpose— to rule out metastatic disease in a patient who otherwise could get local control. It was flatly denied by the Medicare carrier…without any justification.
YU: Yes, it’s been frustrating.
SALGANICK: I don’t know what to do because it’s FDA approved and it’s in the NCCN [National Comprehensive Cancer Network] guidelines, and I still cannot use the test.6 YU: We’ve run into some problems too. We’ve even had patients for whom it’s clearly on-label. We wanted to give the patients lutetium Lu 177 vipivotide tetraxetan, and their insurance company won’t let us give the PSMA PET scan. So that’s frustrating too. All I can say is I think it will get better as time goes on, but keep up the good fight.
SALGANICK: They admitted that it was FDA approved. They just simply said that Medicare carriers can choose to ignore it.
YU: Yes, they can choose to ignore it, but I don’t think that will continue for much longer. If it does, that’s going to be problematic. But it’s good to hear that case though.

KAHN: In my experience, [PSMA imaging] exists, but the problem is getting it approved. I don’t know about anyone else, but I have a hard time getting it approved.
YU: So you put in for the PSMA PET, and the insurance company says, “What’s this?” They don’t know [what it is for]?
KAHN: They just say no.
YU: Yes, it’s frustrating. Dr Zhao, do you have PSMA PET access?
ZHAO: Yes, we do. But as everybody else says, it’s hard to get approved. I personally haven’t gotten anybody tested on that imaging yet.
YOO: It looks like some of the centers are now available to get it done. Before the VISION trial [led to lutetium Lu 177 vipivotide tetraxetan’s approval], I used to send everyone to [clinical trials at] UCLA Jonsson Comprehensive Cancer Center and The University of Texas MD Anderson Cancer Center, [thinking] that this was potentially the future. But after I saw 4 months or 5 months OS, I’m not sure if it’s a magical drug.
YU: Have you ordered PSMA PET for [a patient] yet?
YOO: I’m looking to. I don’t have anyone at this stage yet. Most of my patients are in the castration-sensitive setting.
YU: When would one want to order PSMA PET? It’s approved for essentially 3 different situations. Are people ordering PSMA PET for those earlier situations, like biochemical recurrence or staging? Do you see those patients, or are you just ordering for mCRPC when you’re thinking about giving lutetium Lu 177 vipivotide tetraxetan?
AMBIKA: I tend to order it in biochemical recurrence, and luckily we haven’t had many problems in Nevada so far. I think for anything above 0.5 [ng/mL], the sensitivity is high, from my understanding. So we had a few cases where they approved it.

AMBIKA: I think [the optimal time is at] biochemical recurrence. It’s mainly to see whether we can pick up some oligometastatic disease, and then we can locally ablate, do radiation therapy, and in some cases even stop the ADT after 1 or 2 years if we can control it. So for those kinds of cases, we tend to do that.
YU: It seems like not everyone’s ordered it yet. So it is not unreasonable now, but I think over time, the PSMA PET is probably going to find its way into [greater usage] for prostate cancer. I’d be surprised if it doesn’t.
YU: So this patient had a novel hormonal therapy. Let’s say it’s [abiraterone acetate (Zytiga)] or enzalutamide [Xtandi]. And they’ve had docetaxel, but they just have progression in the bones. What would you do? Does this matter that it’s just progression in the bones and not progression in the liver or [somewhere else]?
VICUNA: For me it matters because I can give radium Ra 223 dichloride as opposed to going to lutetium Lu 177 vipivotide tetraxetan.
YU: If you have this patient and they’re eligible for lutetium Lu 177 vipivotide tetraxetan and...for radium Ra 223 dichloride, do you have a preference one way or another after knowing the data that you know and seeing the data that you’ve seen?
VICUNA: The problem here is at least as of now, we’re not able to get lutetium Lu 177 vipivotide tetraxetan. But assuming both are readily available, I would probably refer to a radiation oncologist because they would be the one giving it. I don’t know if I would have a preference because they’re the ones who must deal with insurance and get approval for radium Ra 223 dichloride or lutetium Lu 177 vipivotide tetraxetan, so I’d probably work with them.
YU: OK, [you would] leave it up to them then. Does anyone else have thoughts on this?
AMBIKA: I thought there were some data showing that it’s safe to do both. Patients who had prior radium Ra 223 dichloride did OK with the lutetium Lu 177 vipivotide tetraxetan, right?
YU: Yes. There are cases where patients have received one after the next and done fine.7 It’s not a large number of patients, and I’m sure that the myelosuppression rates will be higher. It’s just like giving radium 223 and docetaxel or chemotherapy.
You can give one after the next or one before the next. It’s just that of course if you’ve had one, your risk of myelosuppression with the next one is a little higher. But these patients have late-stage disease, so they don’t have a lot of options and everything in life is a risk-benefit ratio. If you’re going to live with a chronic platelet count of 44,000/μL, that’s OK if you’re living in certain situations. Those are the kinds of conversations we all have with our patients.
GALAMAGA: Are there minimum hemoglobin requirements at all for lutetium Lu 177 vipivotide tetraxetan? I believe for radium Ra 223 dichloride there’s a relative recommendation for a hemoglobin of 10 gm/dL and higher.8
YU: [For] a lot of these medications, it’s up to your institution because your institution purchases it in advance. And when they’re really uptight about the criteria, it’s because your institution has a fear that they’re going to get audited later or Medicare is going to ask for a bunch of money back. But…if you use clinical judgment and you think the riskbenefit ratio is in your favor, treat away.
[For] a patient with mCRPC with progression on an AR pathway inhibitor who was deemed ineligible for chemotherapy, it takes cabazitaxel out of the equation there. I think you’re left with either lutetium Lu 177 vipivotide tetraxetan or radium Ra 223 dichloride.
[For] a patient with progression on [an] AR pathway inhibitor after receiving chemotherapy for high-volume mHSPC (metastatic hormone-sensitive prostate cancer), does that affect your opinion at all? Let’s say they received abiraterone and docetaxel, and they’re progressing.
They’ve got a ton of disease. Are you a little bit more nervous about giving lutetium Lu 177 vipivotide tetraxetan or radium Ra 223 dichloride in this situation? Might you lean more toward chemotherapy, or does that not influence your thought process at all?
CHANDAR: In that situation, if I think that the patient is a good chemotherapy candidate and could tolerate cytotoxic chemotherapy, I might consider cabazitaxel just to induce a more dramatic or initial response. I would just be curious to see how the data [pan] out with the lutetium Lu 177 vipivotide tetraxetan moving forward.
YU: In some ways for high-volume disease, I might lean a little more toward chemotherapy. But probably what matters more to me is really the rapidity of the disease. If it’s fastmoving disease, I might lean a little more toward chemotherapy, but I don’t even know that that’s right. So if they have fast-moving, high-volume disease that has low PSMA expression, then yes, that’s probably a patient [who should receive] chemotherapy. But if they uptake PSMA well, I can’t say for sure that lutetium Lu 177 vipivotide tetraxetan isn’t a good alternative for those patients. But it’s certainly a good discussion, and it would be good to see more data in the future.
REFERENCES
1. Pluvicto. Prescribing information. Novartis AG; 2022. Accessed January 19, 2023. https://bit.ly/3Dn2emP
2. Morris MJ, De Bono JS, Chi KN, et al. Phase III study of lutetium177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). J Clin Oncol. 2021;39(suppl 18):LBA4. doi:10.1200/ JCO.2021.39.15_suppl.LBA4
3. Hofman MS, Emmett L, Sandhu S, et al; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, openlabel, phase 2 trial. Lancet. 2021;397(10276):797-804. doi:10.1016/ S0140-6736(21)00237-3
4. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
5. Lutathera. Prescribing information. Advanced Accelerator Applications SA; 2018. Accessed February 21, 2023. https://bit.ly/2MRhWhq
6. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 1.2023. Accessed January 16, 2023. https://bit.ly/3QGSAk4
7. Sartor O, Fougère C, Essler M, et al. 177Lu-prostate-specific membrane antigen ligand after 223Ra treatment in men with bone-metastatic castration-resistant prostate cancer: real-world clinical experience. J Nucl Med. 2022;63(3):410-414. doi:10.2967/jnumed.121.262240
8. Xofigo. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; 2019. Accessed January 19, 2023. https://bit.ly/3CZcTnt