Allan Explores Data on Frontline BTK Inhibition for CLL Treatment
During a Targeted Oncology™ Case-Based Roundtable™ event, John N. Allan, MD, discussed the data supporting the use of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukemia.
John N. Allan, MD
Assistant Professor of Medicine
Division of Hematology and Medical Oncology
Weill Cornell Medicine
New York, NY

Targeted OncologyTM: What data led to the approval of acalabrutinib (Calquence) for the treatment of chronic lymphocytic leukemia (CLL)?
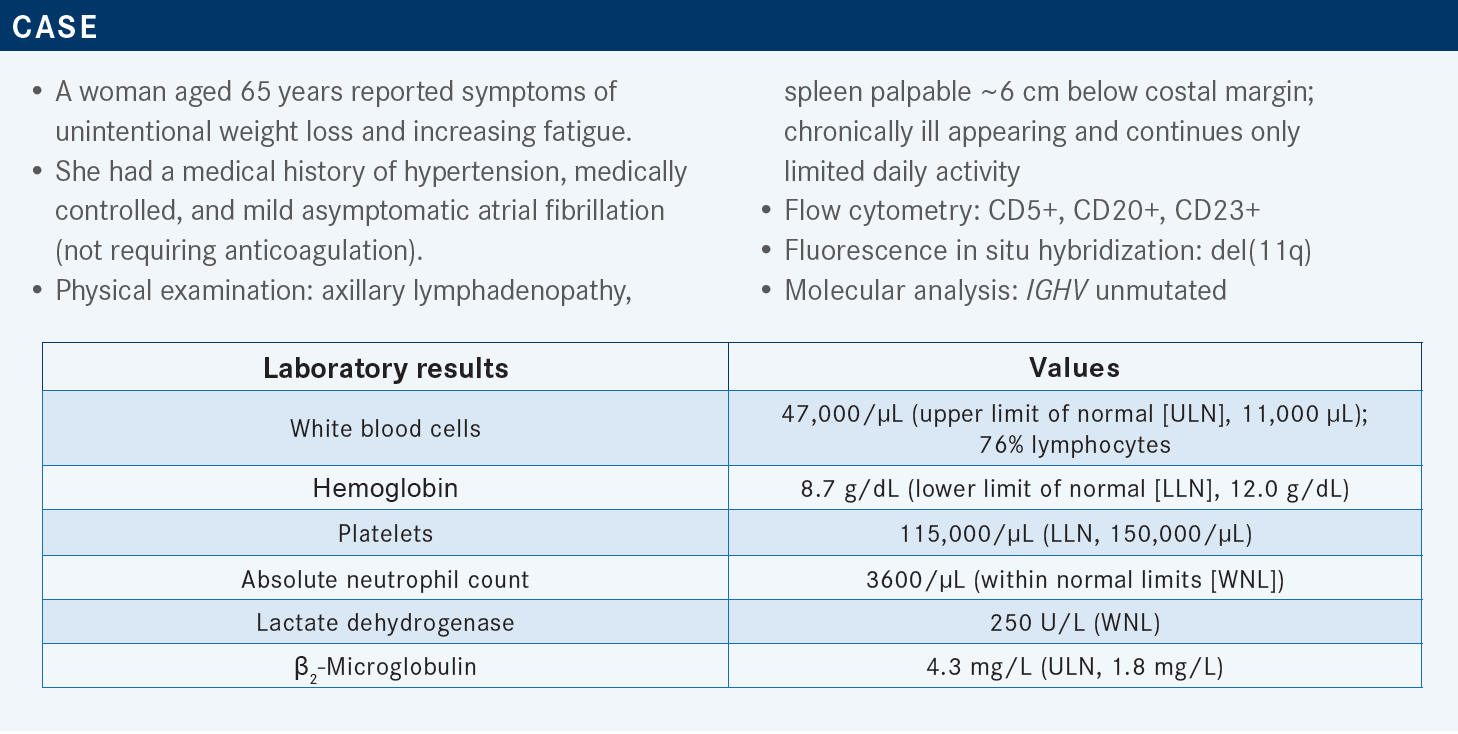
ALLAN: The phase 3 ELEVATE-TN [NCT02475681] study data got acalabrutinib’s approval for use in the frontline setting.1 We’ve now seen 5 years of updates, and [the results were] published in Lancet in 2020.2 This was a study of treatment-naïve CLL in patients [aged] 65 years or more. Almost all Bruton tyrosine kinase [BTK] inhibitor studies done in the frontline setting are in older, frailer patient populations.
In ELEVATE-TN, they were looking at a 1:1:1 random assignment among the study arms of acalabrutinib plus obinutuzumab [Gazyva], single-agent acalabrutinib, and obinutuzumab plus chlorambucil [Leukeran].2
The nice thing about this study is that they had an acalabrutinib monotherapy arm vs the acalabrutinib/obinutuzumab arm, because we knew rituximab [Rituxan] doesn’t seem to add much to ibrutinib [Imbruvica], but we were always wondering [whether] obinutuzumab could add [more]. We do know it is an improved antibody. It does get deeper responses, and in the CLL11 study [NCT01010061] of chlorambucil plus obinutuzumab vs chlorambucil plus rituximab vs chlorambucil, the obinutuzumab arm had improved progression-free survival [PFS], a modest overall survival [OS] improvement, higher complete response [CR] rates, and some minimal residual disease [MRD] negativity. That study established [obinutuzumab], and it’s been shown even in follicular lymphoma to have a benefit over rituximab-based therapies.3 It’s a great agent and it’s very effective. It was unclear [what it] adds to acalabrutinib or to BTK inhibitors in general. The primary end point of ELEVATE-TN was PFS.2
In the updated data at 5 years, the 2 acalabrutinib arms clearly had superior PFS vs the obinutuzumab plus chlorambucil arm. That was what the study was designed to show, but that doesn’t mean much because we knew that already. What is interesting, and what I think has shocked a lot of us and we don’t know what to make of it, is that by the addition of the obinutuzumab [to acalabrutinib], there seemed to be a modest widening of the PFS curves [for the acalabrutinib plus obinutuzumab vs acalabrutinib arms; HR, 0.51; 95% CI, 0.32-0.81; P = .0259]. And mind you, this is just 6 months of standard obinutuzumab treatment, 3 weeks of induction, and then monthly treatment for 6 months, and then it was continuous therapy with a BTK inhibitor. I thought, “How can 6 months of treatment with an anti-CD20 antibody matter, when you’re on a continuous [BTK] therapy?” But we see that there is, in fact, this post hoc PFS benefit by adding obinutuzumab. Now, this study was not prospectively powered to show this difference. So this is descriptive in nature, and it’s hypothesis generating, but every time we do another year of follow-up, that margin [between the acalabrutinib plus obinutuzumab and the acalabrutinib alone arms] widens. It used to be approximately 7%, and then it was approximately 9%, and now it’s up to 12%. That said, that number needed to treat is still high.2,4 A lot of patients are getting anti-CD20 in the era of COVID-19, but you’re starting to wonder [whether] there is some impact here.
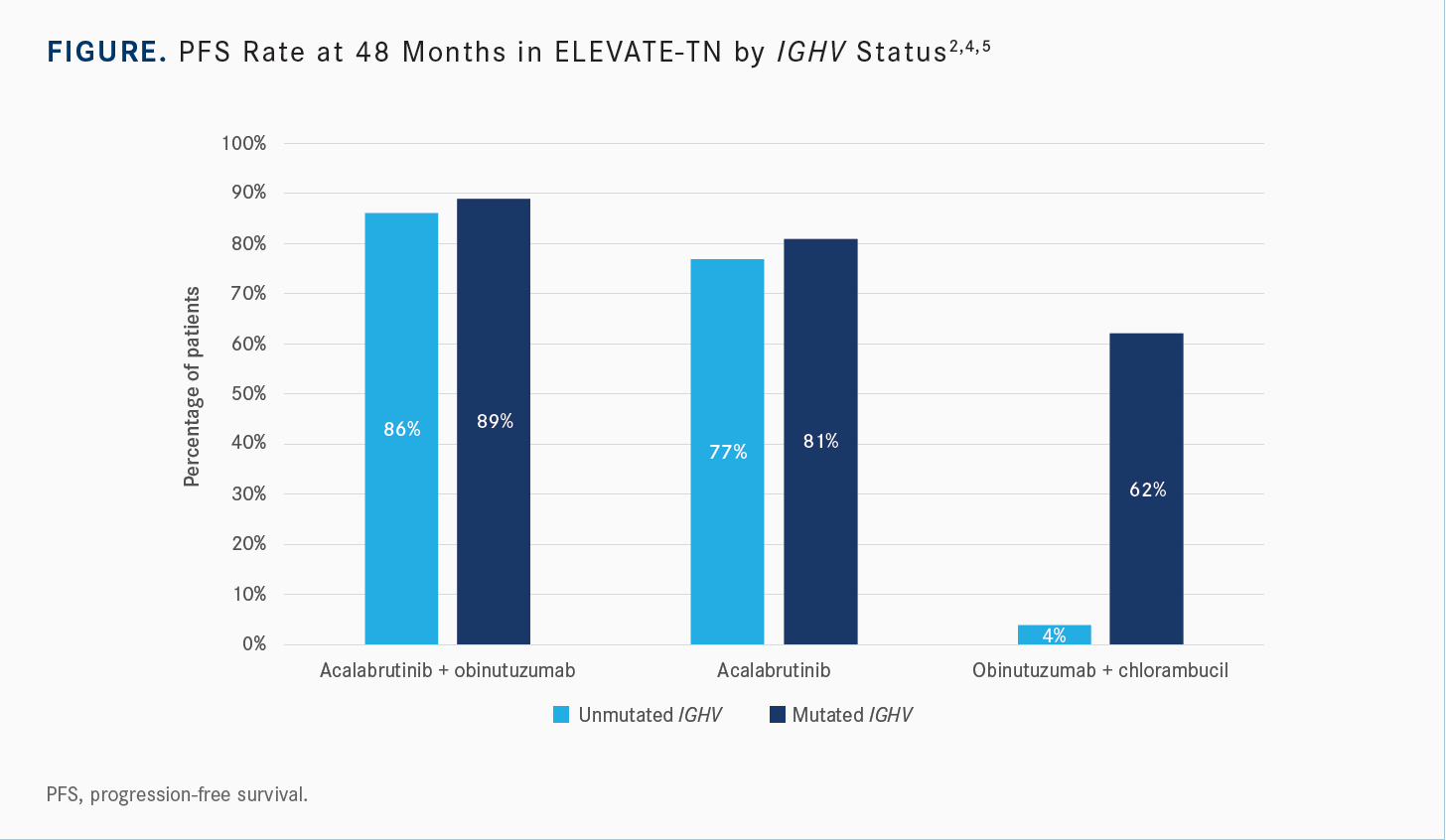
Did subgroup analysis show differential PFS benefit according to IGHV mutational status?
[The data were analyzed] by IGHV mutational status [Figure2,4,5], and what was interesting was that at the early time points, the high-risk patients, [with unmutated IGHV], were not the ones benefiting. [Some experts had made the] argument that if the high-risk patients were [the ones] benefiting, then [the results] made sense. But…the lowest-risk patients, [with mutated IGHV], were the ones who were benefiting early. At later time points, the patients with unmutated IGHV seemed to benefit as well.5
Maybe the reason why we see this difference is that by the addition of the obinutuzumab, you see higher rates of CR, with [32.4%] of patients [in the acalabrutinib/obinutuzumab arm] achieving CR at the 5-year mark [vs 14.5% in the acalabrutinib arm]. We know that as patients are on continuous therapy with BTK inhibitors, [CR rates] can rise to 30% or so over time.6 This [trend] seemed to plateau a bit over time. Close to half of the patients [who achieved CR] achieved an MRD-negative state [42% in the acalabrutinib/obinutuzumab arm, 10% in the acalabrutinib alone arm].
[Very few] of these patients achieved MRD negativity in the acalabrutinib alone arm, but [obinutuzumab] did seem to deepen the response.2,4 And, obviously, if a patient is MRD negative and they come off the drug for [any reason], or even if they stay on the drug, more likely [than] not, that volume of disease is going to take longer to show up than if they don’t [achieve MRD negativity] and then come off therapy or develop resistance.
What OS benefit, if any, was observed among patients treated with obinutuzumab?
[The reason] why I think a lot of physicians are not using the anti-CD20 therapy [obinutuzumab] is that between the 2 acalabrutinib arms, there was no statistically significant difference in OS. However, at [a median follow-up of 58.2 months], there was a statistically significant difference in 5-year OS rate between the acalabrutinib/obinutuzumab and the obinutuzumab plus chlorambucil arms [90% vs 82%, respectively; HR, 0.55; 95% CI, 0.30-0.99; P = .0474].2,4
I argue that the OS in the acalabrutinib monotherapy arm was not too far off [84%], so it makes you wonder [whether], as time goes on, we will start to see an OS [benefit] by the addition of obinutuzumab. Right now, [that benefit] is not there. This study wasn’t powered to show that, so you must take all this with a grain of salt.
But I’m wondering, what if we do start to see an OS benefit? How will this change how we manage our patients? You have to treat a lot of patients with anti-CD20 [therapy], and one of the nice things about this regimen is that it’s an all-oral treatment and you can keep [the patients at] home. They’re not coming into the office on a monthly basis for the initial 6 months. Obviously, the obinutuzumab does add toxicity.
What adverse events (AEs) were observed in ELEVATE-TN?
In the acalabrutinib/obinutuzumab arm, infections of grade 3 or higher affected [28.1%] of the patients vs [19.6%] in the acalabrutinib arm. So, there was some significance in terms of increased infection rates with the addition of the anti- CD20 drug. There was also a lot more neutropenia that was significant, but fortunately it doesn’t seem to turn into febrile neutropenia. Ultimately, there were some toxicities from the obinutuzumab, mostly related to infections. They seem to be mostly managed and have not resulted in death.4,7 But we want to minimize that if we can, and you have to keep that in mind when you are using these drugs, because they do seem to increase the severity of infections.
What data support the use of frontline ibrutinib to treat CLL?
The RESONATE-2 study [NCT01722487] has provided long-term ibrutinib data. This is the frontline study that put ibrutinib on the map. This is a study of treatment-naive CLL in patients who were [aged] at least 65 years [and who] had comorbidities that precluded treatment with FCR [fludarabine, cyclophosphamide (Cytoxan), and rituximab] for patients aged 65 to 69 years. Patients were randomized to either chlorambucil monotherapy or ibrutinib, and patients have now been followed for up to 8 years. This is the longest frontline study with monotherapy BTK inhibitors.8
At a median follow-up of 7.4 years, the median PFS had not yet been met in the ibrutinib arm. Approximately 59% of the patients were progression free [vs 9% in the chlorambucil arm; HR, 0.154; 95% CI, 0.108-0.220]. Just under half of the patients in the ibrutinib arm [42%] remained on therapy. What’s nice to see is that with this long-term follow-up, progressions [while] on therapy were rare and still represented the minority of patients who are progressing.
We’re seeing unbelievable long-term disease control, with [only] 13% of patients progressing on active ibrutinib therapy. The other patients [who] have progressed have come off treatment, for toxicities or for [other] reasons, and stayed on the study and then progressed, 6 months or a year or two later. These responses deepened over time. I don’t know how often they are scanning these patients, but it’s probably at least yearly. [The CR rate is approximately] 35% now, and it does seem to have plateaued a little, but it has deepened during each additional year of follow-up.9
How did ibrutinib perform in patients with high-risk factors in this trial?
BTK inhibitors, ibrutinib in this case, have made high-risk disease features no longer high risk. They still represent high risk in terms of predicting time to first treatment, how patients present, and when they might need treatment after diagnosis. But [regardless of] these features, once you start treatment, every patient is on the same playing field, and we see this over long periods.
There was some concern that at 5 years, the patients with unmutated IGHV were going to continue to progress. But that was not the case. Even at 8 years, the PFS curves [for ibrutinib-treated patients with mutated vs unmutated IGHV] were still overlapping [median PFS, not reached for both groups], whereas among the chlorambucil-treated patients [with mutated vs unmutated IGHV], the PFS curves separated like they always have in the era of chemoimmunotherapy [median PFS, 16.7 months vs 9.3 months, respectively]. So, [regardless of] IGHV mutational status, once you start treatment [with ibrutinib], everybody is on the same playing field.9
That trend didn’t hold just for IGHV mutational status, but also for 11q deletion [del(11q)] status. Patients with del(11q) have always had inferior PFS and OS with chemoimmunotherapy; [this feature] predicts bulky, aggressive disease, [and the] time to first treatment is 2 to 3 years. In RESONATE-2, at the 8-year mark, patients with or without this risk factor were, again, on the same playing field. In fact, in the early time points, up to the first 4 years, the patients with del(11q) were, in fact, experiencing an improved PFS over those without the deletion.9 We need to follow these patients. I can tell you from personal experience, in the 6 or 7 years since I’ve been using these drugs in the frontline setting, many of [my patients] have del(11q), so I have found [this trend] to be true.
How did ibrutinib affect OS?
These patients did phenomenally well, with [78%] of ibrutinib-treated patients still alive at 7 years [HR, 0.453; 95% CI, 0.276-0.743]. From the chlorambucil arm, about half the patients crossed over to ibrutinib.9 [Data have repeatedly] shown that early treatment with these targeted agents confers improved OS compared with chemoimmunotherapy.
What AEs were observed in the RESONATE-2 study?
This study established the class effects of BTK inhibitors. In the ibrutinib arm, atrial fibrillation of any grade affected 6% of patients, hypertension affected 14% of patients, and [upper respiratory tract] infections affected 17% of patients at a follow-up of 17.4 months.8 This [study also] established arthralgias, bleeding, and diarrhea as class effects, but, for the most part, they were low grade and manageable.10,11 Now that we have selective, targeted agents, we’re learning how to use [them] to avoid many of these toxicities, because you can definitely minimize those AEs.
What data support using the combination of ibrutinib plus rituximab instead of FCR?
Those data come from the ECOG-E1912 study [NCT02048813] of ibrutinib plus rituximab vs FCR in a 2:1 randomization. I think [the results of this study are] one of the reasons why the arguments for FCR are going away. There was no monotherapy arm in this study.10
After [a median of 5.8 years] of follow-up, PFS clearly favored the ibrutinib plus rituximab arm [HR, 0.37; 95% CI, 0.27-0.51; P < .0001]. [This benefit] used to be restricted to the IGHV-unmutated group. But now, this [benefit] is also observed in the IGHV-mutated group. So, the group of patients that [historically tended to be] restricted to chemoimmunotherapy are starting to show improved PFS. [This is] more reason to say that [chemoimmunotherapy] is not doing our patients any good.11
There was a modest OS benefit. It should be noted that the companion FLAIR study [ISRCTN01844152], which also examined ibrutinib plus rituximab vs FCR, did not show an OS benefit after approximately 4 years of follow-up.12 But the OS benefit was observed very early in ECOG-E1912, at the first 3-year follow-up time point, and this [benefit] has persisted at 5.8 years’ median follow-up [HR, 0.47; 95% CI, 0.25-0.89; P = .018].11 It’s modest, but there is an OS benefit, which is a reason to question why we are using FCR.
How favorable were the data from the SEQUOIA study (NCT03336333) toward the frontline use of zanubrutinib (Brukinsa)?
This was a big study with 3 cohorts. Cohort 1 is the cohort of interest to the regulatory bodies, because it’s the phase 3, randomized portion of this study. [Keep in mind that] patients with del(17p) were excluded from this cohort, because the patients were randomly assigned to continuous therapy with twice-daily zanubrutinib vs bendamustine [Bendeka] plus rituximab [BR], groups A and B, respectively.
The study’s 2 other cohorts looked at the patients with del(17p), who were excluded from cohort 1. Cohort 2 [group C] is going to be the largest group of patients with del(17p) on any single BTK inhibitor prospective study, and that arm has now shown 2-year data. Cohort 3 [group D] used the fixed-duration approach with zanubrutinib plus venetoclax [Venclexta] for patients with del(17p), with strict stopping rules. The patients had to have achieved a CR and MRD negativity multiple times in blood and bone marrow. These were very strict MRD-negative stopping rules. They enrolled over 80 patients in that cohort, which is starting to show mature data.13
Cohort 1 was restricted to patients with an age of at least 65 years or those with comorbidities [that made them ineligible for FCR]. One patient with a del(17p) mutation [was enrolled], but otherwise, groups A and B were [generally] well balanced [with respect to] high-risk features.14
In cohort 1, PFS favored those patients in the zanubrutinib arm after 2 years of follow-up [HR, 0.42; 95% CI, 0.28-0.63; P < .0001]. Almost all subgroups favored zanubrutinib over BR, just like we see with all other BTK inhibitors.14
Overall response rates also favored zanubrutinib over BR [94.6% vs 85.3%, respectively]. There was a higher CR rate in the BR arm [15% vs 7% in the BR arm], but that obviously doesn’t equate to PFS differences in fixed-duration vs continuous-therapy [settings]. And the patients were still doing well in terms of OS, with similar OS between the arms [94.3% (95% CI, 90.4%-96.7%) vs 94.6% (95% CI, 90.6%-96.9%) in the zanubrutinib vs BR arms, respectively] at this very short time point.14
What AEs were observed among patients treated with zanubrutinib?
The AE profile of zanubrutinib [was characterized by] class effects after 2 years of follow-up. The AEs included [a 19.2% rate of] contusion. There was a relatively low rate of arthralgia of all grades [13.3%] and diarrhea of all grades [13.8%]. Neutropenia seems to be a signal that’s related to zanubrutinib, [with 11.3% of treated patients experiencing grade 3 or higher], but overall it is very manageable.13,14 Some granulocyte colony-stimulating factor can fix this, and it’s not too problematic, honestly.
The AEs of specific interest at 2 years of follow-up included atrial fibrillation [of any grade, affecting 3.3% of patients]. Bleeding rates were similar to those observed with ibrutinib [of any grade, affecting 45.0% of patients].13,14 The rate of diarrhea [of any grade, 13.8%] was maybe a little lower [than that of ibrutinib]; I think ibrutinib at this time point affected approximately 30% to 40% of patients.11
The rate of hypertension [of all grades, affecting 14.2% of patients] was similar to that observed in the ALPINE study [NCT03734016] of zanubrutinib vs ibrutinib [15.7% vs 13.0%, respectively].15 This is something that differentiates zanubrutinib from acalabrutinib a little bit. But even though patients are developing hypertension, they are not developing atrial fibrillation. So, [the hypertension is] not the whole story, but we do want to control it if we can, and it can be controlled.
REFERENCES
1. Project Orbis: FDA approves acalabrutinib for CLL and SLL. FDA. November 21, 2019. Accessed November 28, 2022. https://bit.ly/3IIr7Nf
2. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
3. Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331-1344. doi:10.1056/NEJMoa1614598
4. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: five-year follow-up of ELEVATE-TN. J Clin Oncol. 2022;40(suppl 16):7539. doi:10.1200/JCO.2022.40.16_suppl.7539
5. Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022;36(4):1171-1175. doi:10.1038/s41375-021-01485-x
6. O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919. doi:10.1182/blood-2017-10-810044
7. Gazyva. Prescribing information. Genentech; 2022. Accessed November 28, 2022. https://bit.ly/3iv5RzI
8. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. doi:10.1056/NEJMoa1509388
9. Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6(11):3440-3450. doi:10.1182/bloodadvances.2021006434
10. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. doi:10.1056/NEJMoa1817073
11. Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112-120. doi:10.1182/blood.2021014960
12. Hillmen P, Pitchford A, Bloor A, et al. Ibrutinib plus rituximab is superior to FCR in previously untreated CLL: results of the phase III NCRI FLAIR trial. Blood. 2021;138(suppl 1):642. doi:10.1182/blood-2021-152319
13. Tam CS, Giannopoulos K, Jurczak W, et al. SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab (BR) in patients with treatment-naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood. 2021;138(suppl 1):396. doi:10.1182/blood-2021-148457
14. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031-1043. doi:10.1016/S1470-2045(22)00293-5
15. Hillmen P, Eichhorst B, Brown JR, et al. Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a randomized phase III trial. J Clin Oncol. Published online November 17, 2022. doi:10.1200/JCO.22.00510