Hutson Walks Through Updates in the Management of Clear Cell RCC
During a Targeted Oncology case-based roundtable event, Thomas Hutson, DO, PharmD, discussed the most recent data supporting immune checkpoint inhibitor plus tyrosine kinase inhibitor combinations for frontline treatment of patients with renal cell carcinoma.
Thomas Hutson, DO, PharmD
Director, Urologic Oncology Program
Cochair, Urologic Cancer Research and Treatment Center
Baylor University Medical Center
Texas Oncology-Baylor Charles A. Sammons Cancer Center
Professor of Medicine
Texas A&M College of Medicine
Dallas, TX

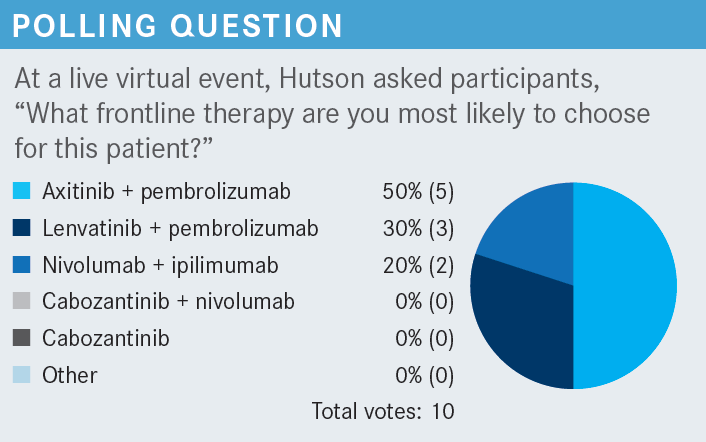
Targeted OncologyTM: What frontline therapy are you most likely to choose for this patient?
HUTSON: The April 2022 National Comprehensive Cancer Network [NCCN] treatment guidelines have [recommendations] for poor- or intermediate-risk patients with clear cell RCC. There is no wrong answer here [because] everything is category 1, outside of single-agent cabozantinib [Cabometyx], which was approved based upon a phase 2 trial in the intermediate- or poor-risk population —and that is likely why it does not have a category 1 recommendation, as it was not a phase 3 study.1 But the FDA has approved it [because] it showed superiority for first-line therapy in patients who are immuno-oncology [IO] intolerant or have a contraindication for IO use.2 Cabozantinib would be certainly high on the list of agents one would choose from.
What data support the recommended frontline regimens for poor- or intermediate-risk patients with clear cell RCC?
The first-line immune checkpoint inhibitor combination trials have updated efficacy data. There’s IO dual combination from the CheckMate 214 study [NCT02231749], which is somewhat unique because it is 2 immunotherapies. The other 3 trials are IO/tyrosine kinase inhibitor [TKI] combinations. So comparing them head-to-head, an IO plus TKI to an IO plus IO is somewhat like comparing apples to oranges a little bit, but that’s what we’ve got to do. Among the IO-plus-TKI trials, cross-trial comparisons would be the most reasonable, and lenvatinib [Lenvima] plus pembrolizumab [Keytruda] is the most recently FDA approved combination.3 The problem is that all 4 trials have different lengths of follow-up, so we have data points that are at various levels of maturity. This has factored into reasons why some physicians may choose one therapy over the other.
The CheckMate 214 [data of] ipilimumab [Yervoy] plus nivolumab [Opdivo] are the most mature, and they’ve published the 5-year outcome data, where patients have maintained durability of benefit 5 years out. Some patients were able to get off therapy, so there’s excitement [about this regimen]. The median overall survival [OS] from the intention-to-treat analysis was 55.7 months vs 38.4 months for the comparator arm [HR, 0.72; 95% CI, 0.62-0.85].4
The HRs for all 4 trials were virtually within the same range, so you can’t separate them out based on HR, except the fact that you have a longer follow-up. The HR for CheckMate 214 is much more mature, and it’s double what’s been reported for the CLEAR study [NCT02811861] and CheckMate 9ER study [NCT03141177]. So will these IO-plus-TKI combinations maintain their HR with a longer follow-up? That is the concern, but across the board, the benefit seems to be similar based on the HR.
The landmark OS [rates] at 12 months and 24 months— and one could say those are very early landmarks—show how things separate out, so it’s hard to show a lot of benefit. The OS [rates] after 24 months all range from 71% to 79%, and the progression-free survival [PFS] rates show differences in HRs for the 4 trials. Whether the length of follow-up is influencing the HR is certainly a point that could be made, and I would argue that it does. You generally lose the HR in PFS over time, as people progress. For instance, CheckMate 214 has a PFS HR of 0.86, which is the worst of the HRs, [whereas] the CLEAR study that has the shortest follow-up and newest agent [has] a very attractive HR of 0.39. For the overall response rates [ORRs], something that’s striking is the CheckMate 214 response rate, which is certainly less—and maybe almost in some situations 40% to 50% less—than what you’re seeing with an IO plus TKI. The complete response [CR] rates look similar, with the CLEAR study having the highest CR rate of 16%. In terms of the progressive disease [PD] rates, the CheckMate 214 [trial] has much higher PD rates than what you see with the other agents.4-7
How would you interpret these data?
It’s difficult to look at this type of data and try to be fair and unbiased...because you’re looking at data...at various levels of maturity. I think what would be fair to say is the dual IO regimen has this long-term follow-up data in which there is a group of patients [appearing] to have durable responses. [About] 20% to 30% of patients who are still on therapy...have had near-CRs or partial responses [PRs] that have been durable, as well as 10% to 12% of people with CRs who may be off therapy.
In about 30% to 40%, or one-third, of patients, the dual IO does not work at all.4 So after the first scan is done, there’s progression. We have a longer follow-up and durability, but it’s not as generalizable; it doesn’t work in as many people. The IO-plus-TKI regimens, on the other hand, work in more people, so you have clinical benefit rates in the 90% range: 90% to 95% with the CLEAR study, about 90% with CheckMate 9ER, and for the KEYNOTE-426 trial [NCT02853331], a roughly 85% clinical benefit rate, which is stable disease, CR, and PR.
They’re going to work in more people, but we don’t have that long-term follow-up to know over time if we are able to maintain responses. Is there going to be any type of flattening of a curve that would suggest that there is a cure possibly? We don’t know that yet with these 3 studies because we just don’t have mature data out yet, so that’s about as fair and unbiased as we can get.

What data support the use of lenvatinib plus pembrolizumab as frontline therapy in advanced clear cell RCC?
The phase 3 CLEAR study had lenvatinib plus pembrolizumab vs everolimus [Afinitor] vs sunitinib [Sutent]. US Oncology Research and Texas Oncology participated in this trial, and one of the authors of the trial was on the steering committee.
The trial was a large international effort, [randomly assigning] patients with advanced clear cell RCC, measurable disease, and good organ function to receive either lenvatinib plus pembrolizumab, at a 20-mg lenvatinib starting dose plus pembrolizumab 200 mg every 3 weeks, with 50 mg of sunitinib given orally on a 4-weeks-on-and-2-weeks-off schedule; or lenvatinib plus everolimus, [at 18 mg of lenvatinib and 5 mg of everolimus]. It was a 1:1:1 3-arm trial, and...the power and statistics were done so that each of the lenvatinib-containing arms were compared with sunitinib—so the lenvatinib arms weren’t compared [with each other].7 Our focus is on the lenvatinib plus pembrolizumab arm vs the sunitinib arm, although lenvatinib plus everolimus fared well against sunitinib, but it is not relevant for us in the frontline setting.
The primary end point of the study was PFS, and secondary end points included OS, ORR, safety, and health-related quality of life. Some exploratory end points were duration of response and identifying biomarkers. There was prespecified stratification [of patients on the trial] into favorable-, intermediate-, or poor-risk categories, as well as geographic region.
The primary end point of PFS at a median follow-up of 26.6 months was 23.9 months for lenvatinib plus pembrolizumab vs 9.2 months for sunitinib [HR, 0.39; 95% CI, 0.32-0.49; P < .001], with the difference at 14.7 months.7 This is the greatest PFS that we’ve ever seen of a therapy in the frontline setting in kidney cancer. The PFS results favored the 2 combination regimens over sunitinib across all the evaluation end points, including Memorial Sloan Kettering Cancer Center [MSKCC] or the International [Metastatic RCC] Database Consortium [IMDC] risk groups.
How does the subgroup analysis impact the implications of these data?
The subgroup analysis by blinded review had several baseline demographics that showed if any prespecified stratification criteria, including adverse prognostic features, benefited lenvatinib plus pembrolizumab or not. Across the board, everything benefited lenvatinib plus pembrolizumab; irrespective of age, sex, and geographic region, lenvatinib plus pembrolizumab was superior.7
What’s great for us as oncologists, irrespective of the IMDC or MSKCC risk group, you don’t have to calculate their risk scores. This drug will work across the board in all the risk groups. You don’t need to worry. Subgroups based on performance status, PD-L1 expression, sites of metastases, prior nephrectomy, sarcomatoid histology, liver metastases, and bone metastases all benefited equally with the lenvatinib plus pembrolizumab.
The OS at median follow-up of 26.6 months showed a separation of curves in favor of lenvatinib plus pembrolizumab over sunitinib. There is crossing later, but that’s a small number of events that are not statistically valid. The HR is very attractive at 0.66 in favor of lenvatinib plus pembrolizumab over sunitinib [95% CI, 0.49-0.88; P = .005].7 Virtually all these therapies are sitting in that 0.7 to 0.8 HR with long follow-up. In fact, the 5-year follow-up with ipilimumab plus nivolumab is sitting at 0.72. This was an earlier analysis, so it’s showing better, but it does climb into the 0.7 range [HR, 0.72; 95% CI, 0.55-0.93] with the 33.7-month median follow-up data, which were presented at the Kidney Cancer Research Summit in 2021, but the median OS hadn’t been reached.8
For the ORRs, there is a high CR rate of 16.1% for the lenvatinib-plus-pembrolizumab arm, with a very low progressive disease rate. So roughly 94.6% of patients having at least stable disease—that’s the clinical benefit rate. It is very striking to give a therapy and know on the first scan that there’s a 95% chance they’re at least going to have stable disease. So one can choose it and feel comfortable that there’s going to be at least that level of activity.
The median duration of response that’s been shown so far is 25.8 months in those that are responding; 79% maintained a CR at 24 months, and 74% maintained a CR at 36 months.7 We need to see the durability at 60 months when compared with the ipilimumab-plus-nivolumab data, and we just don’t have that yet. It still looks good at 36 months, but you’re losing the patients and getting CRs that are failing over time.
What toxicities should be highlighted from this treatment?
Based on treatment exposure, safety, and discontinuation, lenvatinib plus pembrolizumab is a very tolerable regimen, although it does require some work on both the patient’s side and the physician’s side. The adverse effects [AEs] need to be handled because almost 100% of people are going to have treatment-related AEs, and those greater than or equal to grade 3 are over 80%. One then needs to consider dose reductions. And in the trial, the lenvatinib-plus-pembrolizumab group had roughly 70%, or two-thirds, of patients dose reducing. So one goes into the study starting at 20 mg, but with the recognition that two-thirds of the time you’re going to have to lower the dose.7
Toxicities are handled by dose interruption and dose reduction. If done, one can keep most patients on the regimen. So although [70% of people require] dose reductions, the majority can stay on therapy at a lower dose and continue to get benefit. Remember, we are comparing a combination regimen with a single agent, so you do get a slight increase in toxicity, but it’s not overwhelming. Grade 3 or 4 toxicities are a little bit more [frequent] with the lenvatinib plus pembrolizumab, but not drastically so.7 There is added toxicity with the combination, but it’s still within a reasonable range, given the amount of efficacy benefit you get with the therapy.
Are you familiar with the recommended dosages for lenvatinib?
I was fortunate enough to be good friends with Robert J. Motzer, [MD, of MSKCC], and he called me up years ago when E7090, which was lenvatinib, was entering its phase 1 trial. We did the phase 1 trial with it plus everolimus back then. So I was involved a little bit with the pharmacology and the drug development. It was shown that the higher the dose, the better it inhibits the FGFR pathway.
One of the reasons we think lenvatinib does so well in its mechanism of action and in resistant patients is because it inhibits FGFR. So there’s this dose response of wanting to try to hit 20 mg. There was also a phase 2 study performed by Sumanta K. Pal, [MD, of City of Hope,] that was presented this past year and a half, where they looked at lenvatinib plus everolimus, and they tried to compare outcomes of starting lenvatinib at 14 mg vs 18 mg.9 They found that 14 mg was inferior to just starting at the higher dose and going down. With all that said, the theory of inhibiting receptors plus the clinical data suggests a dose response, so the thought is to try to get most of your patients to start at 20 mg.
To be honest with you, if you start at a lower dose, it’s hard to go up. One could go up in some, but some are having AEs, and they wouldn’t want to go up. So I find it better to start up and then go down. Don’t wait for the patient to be miserable. Inform them ahead of time that the reason you’re doing this is to try to get optimal shrinkage. Then most of the time, they’re good with that. The way it’s dosed down is 20 mg to 14 mg to 10 mg to 8 mg; then after completion of 2 years, pembrolizumab is discontinued, and then the patient stays on lenvatinib until progressive disease or toxicity—although people are entertaining the idea that patients with near-CRs or prolonged durable responses can have lenvatinib scaled back too. That’s an area of ongoing study. The dosage forms are the 4-mg and 10-mg capsules.10
What data support the use of nivolumab plus cabozantinib in frontline therapy for advanced clear cell RCC?
The CheckMate 9ER study uses the other newer regimen that’s come out in the past year. It was an international study with 650 patients with untreated, advanced, or metastatic clear cell RCC. Like the other IO-plus-TKI trials, it involves all IMDC risk groups, so favorable, intermediate, [and] poor. Stratifications were based upon that, as well as tumor PD-L1 expression and geographic region. The randomization is 1:1, with 240 mg of intravenous nivolumab every 2 weeks and 40 mg of oral cabozantinib. Sunitinib was the traditional dose of 50 mg daily on the 4-weeks-on-and-2-weeks-off schedule. The primary end point was PFS, and secondary end points were OS, ORR, and safety. They presented their median follow-up data at the earliest time point of any of the trials after an 18.1-month median follow-up.
What are your reactions to these data?
The PFS is striking, and there’s a clear separation of the curves [when you look at the data presented as a graph]. The median PFS after 18.1 months of follow-up was 16.6 months for the combination vs 8.3 months for sunitinib [HR, 0.51; 95% CI, 0.41-0.64; P < .0001].6 The 33-month median follow-up was reported recently at the February 2022 Genitourinary Cancers Symposium by Toni K. Choueiri, [MD, of Dana-Farber Cancer Institute,] and the PFS was again 16.6 months vs 8.3 months [HR, 0.56; 95% CI, 0.46-0.68].11
There is a little better benefit with the combination in patients with bone metastases. Whether patients had nephrectomy or not, there was a benefit, so it seems it was similar across the other IO plus TKIs in that regard. For the OS at 24 months, there is an absolute 10% improvement in survival, and the OS after a 33-month follow-up was 37.7 months for the combination and 34.3 months for sunitinib [HR, 0.70; 95% CI, 0.55-0.90].11 Remember, lenvatinib plus pembrolizumab had a hazard ratio 0.72 after a 33-month follow-up, and then the hazard ratio was 0.72 at the 60-month follow-up for ipilimumab plus nivolumab, which is again in that range. The question is what happens over time.
For ORR, there is a difference in the CR and PR rates. If you add stable disease into that, you’re getting close to a 90% clinical benefit rate. The CR rate was 8% for the combination vs 4.6% with sunitinib, and it was a statistically significant difference. The median duration of response was 20.2 months for the combination and 11.5 months for sunitinib. The median time to response was 2.8 months for the combination and 4.2 months for sunitinib.6,11
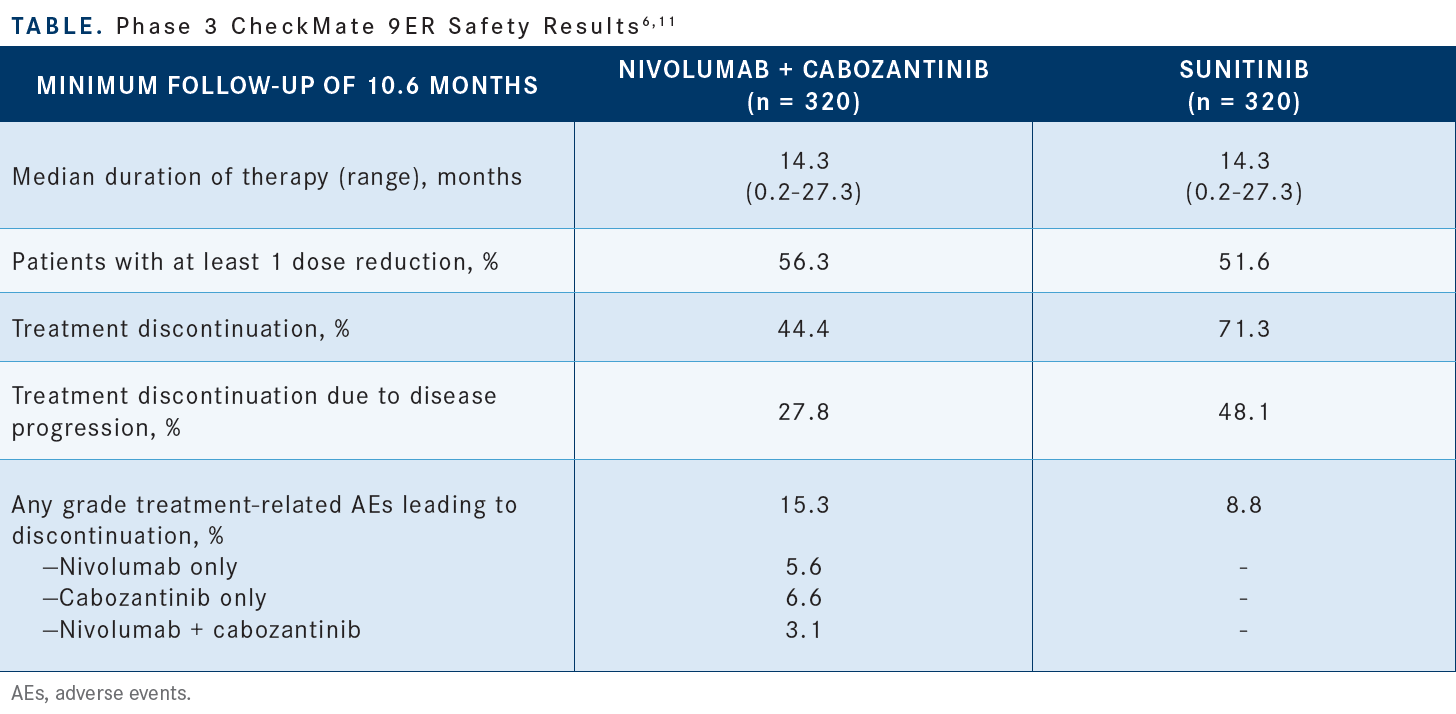
The treatment discontinuation rate was lower with the combination than it was with sunitinib [Table6,11]. [More patients on the combination] had at least 1 dose reduction [compared with] sunitinib. It wasn’t as high as the 70% we saw with lenvatinib plus pembrolizumab, but there are a fair number of patients, almost 60%, who required a dose reduction. Like with lenvatinib plus pembrolizumab, dose reductions and dose interruptions were the main means of handling AEs. If dose reduction was done, the discontinuation rate of nivolumab plus cabozantinib was like that of lenvatinib plus pembrolizumab.
References
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2022. Accessed July 6, 2022. https://bit.ly/3tQpaWN
2. FDA grants regular approval to Cabometyx for first-line treatment of advanced renal cell carcinoma. FDA. Updated December 19, 2017. Accessed July 6, 2022. https://bit.ly/3yN26uV
3. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. Updated August 11, 2021. Accessed July 6, 2022. https://bit.ly/3N7yGLN
4. Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab vs sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085-2097. doi:10.1002/cncr.34180
5. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib vs sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. Published correction appears in Lancet Oncol. 2020;21(12):e553.
6. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib vs sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
7. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
8. Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab vs sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (the CLEAR study). Presented at: Kidney Cancer Research Summit 2021; October 7-8, 2021; Philadelphia, PA. Abstract E41. Accessed July 18th, 2022. https://bit.ly/3yRcxMx
9. Pal SK, Puente J, Heng DYC, et al. Assessing the safety and efficacy of two starting doses of lenvatinib plus everolimus in patients with renal cell carcinoma: a randomized phase 2 trial. Eur Urol. Published online February 21, 2022. doi:10.1016/j.eururo.2021.12.024
10. Lenvima. Prescribing information. Eisai Inc; December 2021. Accessed July 6, 2022. https://bit.ly/3tSHiiB
11. Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib vs sunitinib for patients with advanced renal cell carcinoma. J Clin Oncol. 2022;40(suppl 6):350. doi: 10.1200/JCO.2022.40.6_suppl.350

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen