Couriel Assesses the Available Data for Treatment of Acute GVHD
During a Targeted Oncology case-based roundtable event, Daniel R. Couriel, MD, MS, MBA, discussed how to predict risk of acute graft-vs-host-disease and manage its effects on patients with hematologic malignancies.

Daniel R. Couriel, MD, MS, MBA
Director, Utah Blood and Marrow Transplant Program
Huntsman Cancer Institute
Salt Lake City, UT

Targeted OncologyTM: What is this patient’s risk of developing acute GVHD (aGVHD)? What are other risk factors for aGVHD?
COURIEL: The risk factors can be classified as donor-recipient factors, stem cell graft factors, and transplant factors. [For each of these factors, specific] conditions can increase the risk of aGVHD.
Donor-recipient factors include major human leukocyte antigen [HLA] disparity, mainly class I and class II, with a greater risk associated with mismatched than with matched donors. [There was also] minor HLA disparity, greater risk with unrelated than with related donors; sex matching, greater risk with mismatched than with matched donors; donor parity, with a greater risk with multiparity than with nulliparity; and donor age, greater risk with older donors than with younger donors.
[This effect of donor age] is even more truthful for certain types of transplants, like haploidentical transplants, with older donors infrequently [responding] better than younger donors. For ABO blood type, a greater risk is associated with mismatched donors than with matched donors, and for donor CMV serostatus, positivity is associated with greater risk than is negativity. Numerous cytokine gene polymorphisms have been associated with aGVHD, but the problem with [identifying] these [polymorphisms] is that [doing so requires many] patients, and we deal with diseases that are not [common].
Stem cell graft factors [include] the stem cell source; peripheral blood [stem cell] grafts [are associated with a greater risk than are] bone marrow grafts, which, in turn, are associated with a greater risk than are umbilical cord [blood grafts]. For graft composition, a higher CD34+ count [and a higher T-cell dose] are both more likely to cause aGVHD than are a lower CD34+ count [and a lower T-cell dose]. Finally, conditioning intensity, a transplantation factor, affects aGVHD risk, and myeloablative regimens are associated with greater risk than are reduced-intensity nonmyeloablative regimens.
What GVHD staging and grading system is preferred for everyday use? How has organ staging been applied to risk stratification?
That preferred staging and grading system is the Mount Sinai Acute GVHD International Consortium [MAGIC] system, which is largely based on the Glucksberg system. [The MAGIC system assigns stages for] skin, liver, upper gastrointestinal [GI] tract, and lower GI tract. There are only minor differences in comparison with the Glucksberg system.1
A GVHD risk stratification system, [based on staging and] mostly intended for clinical research, was published a few years ago by [Margaret] MacMillan, MD, MSc, from the University of Minnesota. I like the simplicity [of this system], which splits patients into standard-risk and high-risk groups.
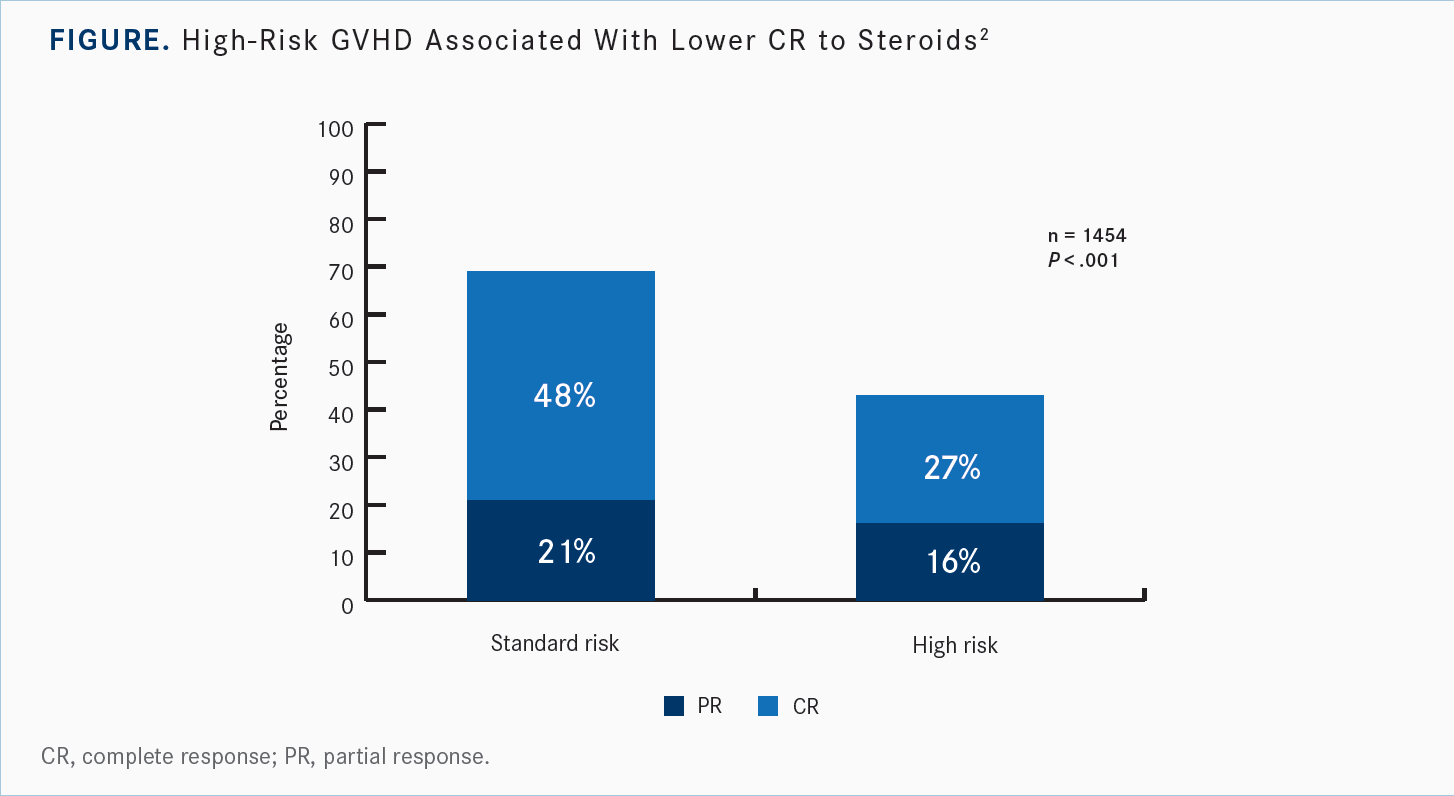
[The standard-risk classification applies to] stage I-III skin, stage I-II GI, stage I-III skin plus stage I GI, and stage I-III skin plus stage I-IV liver, and everything else is high risk. [I think] it works nicely from a clinical research perspective.2 Standard-risk and high-risk patients, [as defined by this system], exhibit dramatic differences in the cumulative incidence of transplant-related mortality at 6 months at 22% [95% CI, 20%-24%] vs 44% [95% CI, 38%-50%], respectively. There are also significant response rate differences, especially the complete response [CR] rate [at 48% vs 27%, respectively], along with lower CRs to steroids [Figure2].

How do you define steroid-refractory aGVHD?
[The definition that] I use was used in the REACH2 study [NCT02913261] of ruxolitinib [Jakafi]. I thought that this was probably the best definition, encompassing everything that I’ve worked with or thought about throughout the years.
According to this definition, steroid-refractory aGVHD includes GVHD that has either increased in stage in any organ system or demonstrated new organ involvement, after 3 days of primary treatment with methylprednisolone [Medrol] at a dose of at least 2 mg/kg daily.3 This part of the definition is something that I initiated a long time ago in my first infliximab [Remicade] study, where I noticed [that those who] progress are not the same as [patients who do not] respond and keep their stage.
[The definition of steroid-refractory aGVHD also includes] GVHD that has not improved, such as if it has not decreased in stage in at least 1 involved organ system, after 7 days of treatment with methylprednisolone at a dose of at least 2 mg/kg daily. Additionally, patients who previously began corticosteroid therapy at a lower dose, at least at 1 mg/ kg daily, for treatment of skin GVHD or skin GVHD accompanied by upper GI GVHD, but who develop new GVHD in another organ system, were also included.3
This third category represents something that shouldn’t have happened. Why does a person who has both skin and GI GVHD start with 1 mg/kg per day? If such a patient had been treated adequately, with 2 mg/kg, you might not have been confronted with the situation of steroid refractoriness, which has a mortality [rate] of up to 80%.4
The final category [of steroid-refractory aGVHD] is [represented by] patients who cannot tolerate corticosteroid taper; that is, they begin corticosteroids at 2 mg/kg, demonstrate a response, and progress before a 50% decrease from the initial starting dose of corticosteroids is achieved.3 Some people [call this] steroid dependence, but to me it’s semantic.
I do not [regard] 7 days of 4 L of diarrhea [per day] as the same as 7 days of rash that covers 60% of the BSA. When GVHD is visceral, my tolerance for stability may not be 7 days. That’s 1 thing that I add between parentheses. So, if you’re confronted with that in practice, you probably want to do something before you reach day 7.
What does the National Comprehensive Cancer Network (NCCN) recommend for the treatment of patients with steroid-refractory aGVHD?
There is a long list of NCCN recommendations for the treatment of aGVHD.5 The reason [for the long list] is that the NCCN is used by payers to decide whether or not they cover [one of the listed treatments for a treatment in the] second line. The intention [in creating the long list] is to make everyone’s life easier. So, when [anyone goes to] look at these guidelines, they should take the time to read the text, because that’s where the [substance] is. According to the guidelines, ruxolitinib, approved for steroid-refractory aGVHD,6 is the best second-line therapy.5
Upon what data was the approval of ruxolitinib based?
[Those data came from the] phase 2 REACH1 study [NCT02953678] of ruxolitinib for steroid-refractory patients with aGVHD. The eligibility criteria were, basically, [the same as the] definition of steroid-refractory aGVHD, [as I previously mentioned]. The primary end point was overall response rate [ORR] on day 28, and there were [several] secondary end points. Seventy-one patients were treated [with 5 mg of oral ruxolitinib] twice a day. [Patients also received] steroids, with or without a calcineurin inhibitor, while ruxolitinib acts through the inhibition of STAT1 [and] STAT3, and both are broadly immunomodulatory.3
[In the REACH1 study], the ORR on day 28 was 54.9%, and the best ORR [at any time during treatment] was 73.2% with the rates of CR at [26.8% at day 28; 56.3% at any time during treatment] were considerable for steroid-refractory aGVHD. The median time to response was 7 days [range, 6-49]; the median duration of response was 345 days. The nonrelapse mortality [rate] at 6 months was [44.4%].7 That is quite good, [considering] that 15 to 20 years ago we used to have mortality [rates] of up to 80% at 6 months, while on the trial the median overall survival [OS] for people who responded on day 28 was not reached.
What were the results of the phase 3 trial of ruxolitinib?
REACH2 [NCT02913261] was the phase 3 [trial of ruxolitinib] for aGVHD. [In this trial], the dose was 10 mg oral twice a day, and [the experimental therapy] was compared with the best available therapy, selected by the investigator prior to randomization. The primary end point was, again, ORR on day 28, and there was long-term follow-up until month 24.3 The ORR on day 28 was 62.3% for ruxolitinib and 39.4% for the control group [odds ratio, 2.64; 95% CI, 1.65-4.22; P < .001)]. [There was] a nice CR rate, significantly higher for the ruxolitinib arm at 34.4% [vs the control arm at 19.4%]. The durable overall response rate at day 56 was, again, significantly higher in the ruxolitinib arm than in the control arm [at 39.6% vs 21.9%, respectively (odds ratio, 2.38; 95% CI, 1.43-3.94; P < .001)], and this was particularly notable in CRs at [26.6% vs 16.1%, respectively].8
The responses on day 28 [were analyzed for subgroups defined by] stage at randomization. [Patients who started with] a lower grade tended to have better responses. [There was] a CR rate of [50.9%] for grade 2 patients, [28.2% for grade 3], and [20.0%] for grade 4. [The same pattern was observed in the control arm] but with mostly lower values for both CRs and partial responses. Additionally, there was a dramatic difference between the ruxolitinib and control arms with respect to response duration.
The failure-free survival for ruxolitinib at 6 months was about 50%, which is good, because the best available therapy was less than 40%. The adverse events [included] cytopenias, particularly thrombocytopenia at 33% in the experimental arm vs 18% in the control arm, and anemia at 30% vs 28%, respectively. Also, cytomegalovirus infections were notable, but this was not dramatically different between groups [at 26% vs 21%, respectively]. The thrombocytopenia, though, was [dramatically different between groups].8
REFERENCES
1. Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. doi:10.1016/j.bbmt.2015.09.001
2. MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. doi:10.1016/j.bbmt.2015.01.001
3. Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff NV. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391-402. doi:10.2217/imt-2017-0156
4. Česen Mazič M, Girandon L, Kneževič M, Avčin SL, Jazbec J. Treatment of severe steroid-refractory acute-graft-vs.-host disease with mesenchymal stem cells-single center experience. Front Bioeng Biotechnol. 2018;6:93. doi: 10.3389/ fbioe.2018.00093
5. NCCN. Clinical Practice Guidelines in Oncology. Hematopoietic cell transplantation (HCT), version 1.2022. Accessed July 14, 2022. https://bit.ly/3uYeUw7
6. FDA approves ruxolitinib for acute graft-versus-host disease. FDA. May 24, 2019. Accessed July 14, 2022. https://bit.ly/310cHje
7. Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. doi:10.1182/blood.2020004823
8. Zeiser R, von Bubnoff N, Butler J, et al; REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. doi:10.1056/NEJMoa1917635