Roundtable Discussion: Barriers to CAR T-Cell Therapy for DLBCL Lead to Debate About Targeted Therapy
During a Targeted Oncology case-based roundtable event, Pallawi Torka, MD, discussed with participants when to consider a patient for chimeric antigen receptor T-cell therapy and what alternative treatments to use for a patient with relapsed/refractory diffuse large B-cell lymphoma.
Pallawi Torka, MD (Moderator)
Assistant Professor of Oncology
Lymphoma Section, Department of Medicine
Roswell Park Comprehensive Cancer Center
Buffalo, NY


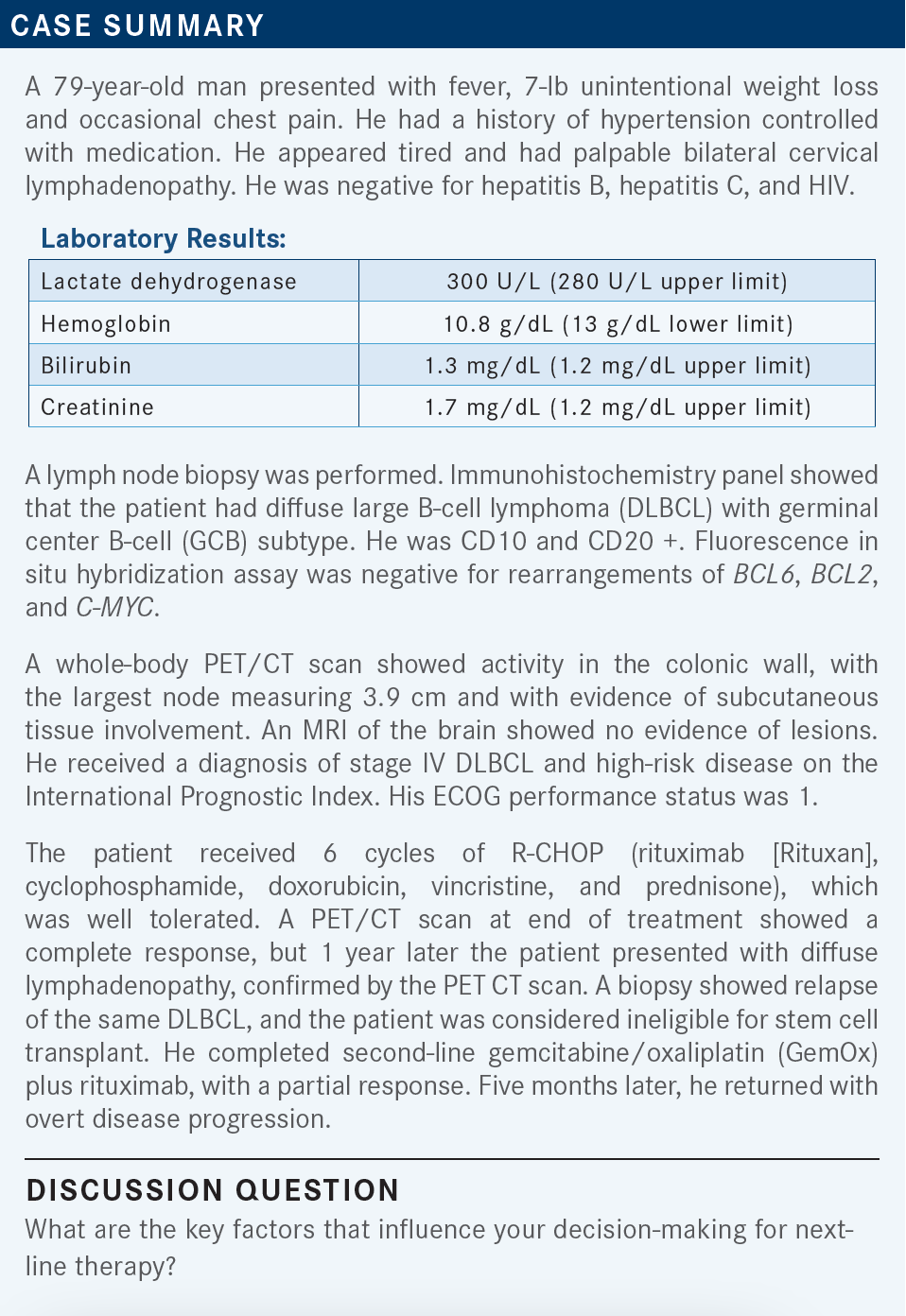
KALAVAR: I consider the age of the patient, comorbidities, if they are candidates for transplant or CAR [chimeric antigen receptor] T-cell therapy, prior regimen given, and patient choice. If I must send the patient for CAR T-cell therapy, I consider the logistics and availability. Most likely a 79-year-old patient is not a candidate for transplant. I would consider CAR T-cell therapy for this patient, [depending on how] willing he is to travel to a different center for it. These are all the things [I think about] before I make the final decision.
Of course, [I think about] insurance coverage too. But my last patient had no problems having the insurance cover it. There is a delay between securing the CAR T-cell therapy [and receiving treatment], depending on how sick the patient is and how urgent the treatment is.
STEINBERG: This seems like a patient who, if you can get [them] referred for CAR T-cell therapy at this age, could tolerate it. That would be the best approach or best chance for long-term remission. But for whatever reason— if they are too far away, it’s not logistically going to work for them, or if they have poor social support—I’d probably consider polatuzumab vedotin [Polivy].
TORKA: Do you, in the relapsed/refractory setting, think about how cell of origin could be leveraged in terms of therapy? Is that a consideration at all?
STEINBERG: For me, it helps more with prognosis when I talk to the patient, worry about them, or watch them more carefully. But I don’t know if it’s necessarily going to affect my management unless I was trying something off-label, such as abivertinib [Fujovee], maybe, in germinal center B-cell (GCB) lymphoma.
TORKA: Dr Sood, are there any differences in your practice because it’s more in western New York compared with many of our guests who are from closer to [New York City]?
SOOD: My enthusiasm for CAR T-cell therapy has waned over the years [because] the data show it doesn’t seem to be as promising as just chemotherapy. Also, there is the factor of getting the patient back and forth.
I think my preference would be the tafasitamab [Monjuvi] plus lenalidomide [Revlimid] combination….I can deliver it in the community setting, and it seems tolerable. For a 79-year-old patient, I think the outcomes are similar. I would choose [tafasitamab/lenalidomide] for this patient.
TORKA: I think the general sense in the community is initially that there was so much enthusiasm about CAR T-cell therapy, and it is good for a subset of patients, but it hasn’t proved to be beneficial for most patients.
How do you choose among these options for patients?
HERNANDEZ-ILIZALITURRI: Age, performance status, and organ function are important in selecting therapy, but the other thing is the time of relapse. Because patients who relapse within 1 year of completing R-CHOP are more likely to be chemotherapy-refractory to a chemotherapy-based regimen. For those patients, I would be more inclined to use an immunotherapy; CAR T-cell therapy, if they can qualify for it socially, financially, and physically; or tafasitamab plus lenalidomide.
Patients who relapse after 1 year most likely are chemotherapy-sensitive. Whether you pick a dose-intense regimen plus consideration of transplant, or a chemotherapy-based regimen such as pola-BR [polatuzumab, bendamustine, and rituximab], or loncastuximab [Zynlonta] depends on the age and the performance status of the patient. To me, if a patient is not a candidate for a bone marrow transplant, chances are they are not candidates for CAR T-cell therapy, because they pretty much require the same qualifications.
TORKA: I want to challenge you on that a little bit. I agree that a majority of the patients do share the same eligibility criteria. However, patients on CAR T-cell therapy studies were even enrolled up to the age of 86, and they could have more organ function compromise compared with those who go into transplant.
With CAR T-cell therapy, patients with active disease can get it. So if they have organ compromise because of active disease, it’s acceptable. But most patients who go to transplant, go in with complete remission [CR], so it is more during consolidation. Although most patients do share the same criteria, which makes them ineligible.
Patients [I’ve seen] who have aggressive disease never make it to CAR T-cell therapy because we don’t have the time. We keep going from treatment to treatment and are never able to get the disease controlled enough to do the CAR T-cell therapy. Has anybody had similar experiences?
STRAUSS: I’ve had experience with patients who get so sick and their relapsing goes fast, while they’re inpatient, and you aren’t going to ship them off from inpatient to CAR T-cell therapy. One gives something a little bit more urgent, something that you can get right away.

TORKA: Now with all these approvals, it is possible that many of the patients get an idea of what can be done in the second or third line [at the academic centers], then the treatment is delivered back in your office.
Is that what has been happening in many cases? Does the academic center suggest treatment and the patients come back, or do they just stay at the academic center? Any comments on that from community oncologists?
STRAUSS: It’s mixed. Sometimes they are enamored by the academic institution and decide to stay there. On the flip side, sometimes they go there, and they’d rather go down the street to a smaller practice where you park, you take 2 steps, and you are in the waiting room. Both have a different vibe, and some patients prefer one, and some patients prefer the other. I’d say it’s half-and-half. But if it’s something that I can give in the community setting, I give it.
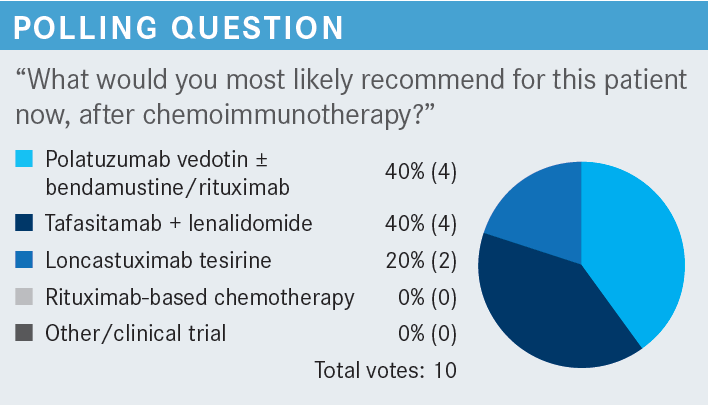
SOOD: What is the response rate of single-agent polatuzumab? What is the benefit of adding BR [bendamustine/ rituximab] to it to begin with?
TORKA: Single-agent polatuzumab is not very active. I would say in the initial studies it had overall response rates [ORRs] of [approximately] 20% combined in all lymphomas. Every lymphoma was a small single-digit number. If you are asking for DLBCL, specifically, I don’t know. I tried to look for studies where polatuzumab plus rituximab was given, but there is not much data for that. Although, in clinical practice, it is potent. If I have an older patient who I don’t want to give bendamustine, I start out with polatuzumab plus rituximab for 1 cycle to see how they do, and then add the bendamustine once they feel a little bit better. It works.
HERNANDEZ-ILIZALITURRI: I think it’s important to be reminded that BR alone is not a regimen for DLBCL. The study [looking at polatuzumab vedotin with rituximab or obinutuzumab (Gazyva) plus bendamustine (NCT02257567)] was highly criticized because of the backbone [of BR alone]. But the 3 drugs together is an approved regimen.
TORKA: If I did not have polatuzumab available, I would not use BR. I would rather go to GemOx. This patient got GemOx as second-line therapy, not BR. I don’t think there’s any justification in giving BR alone to patients.

STEINBERG: The main thing that has limited my use of it is getting lenalidomide. It’s such a hassle because of the co-pay and all the other stuff you go through. I’ve used it, but it’s almost like I use single-agent tafasitamab while waiting for lenalidomide to arrive, and it takes forever, so it increases my dissatisfaction with using it. That’s why, for example, I prefer the treatments that are IV [intravenous] that you can give every couple of weeks, such as polatuzumab or loncastuximab.
HERNANDEZ-ILIZALITURRI: That’s a valid point, especially because lenalidomide alone does not have an FDA level approval for DLBCL. On the other hand, if you look at its long-term data from the L-MIND study [NCT02399085] of tafasitamab/lenalidomide, the patients who respond tend to sustain remission for a long time—more than 4 years.1 The number of patients who are in that bracket is much higher than those on loncastuximab or pola-BR. So if you find the correct patient, I think it is worth the extra paperwork.
TORKA: Both points are valid. At academic cancer centers, we are spoiled because we have whole teams that are working on the prior authorizations and such. What about the other community practices? Is [difficulty] getting lenalidomide approval something that is commonly experienced in the community?
SOOD: For me, it’s not good in the community setting. But, in general, it’s not a huge barrier. If you believe in it, I think you can get it done.
STEINBERG: In general, getting the oral drugs is more difficult because they require co-pay assistance, and you must go through the REMS [Risk Evaluation and Mitigation Strategy] program for lenalidomide, which is a little bit of a different process than for something we give IV. When it is something IV, the hospital runs our infusion center, so we just order it, and that’s all done on the back end, and it’s not something that we as a practice worry about. The oral drugs are a little trickier and can potentially be more costly to the patient.
TORKA: Dr Mehta, do you have pharmacists to help you with the REMS program and such, or is it all falling on the practitioner?
MEHTA: Yes, we have pharmacies, but most of the responsibility is on the physicians. We take care of that, but they help us in preparing the chemotherapy and immunotherapy.
TORKA: So all the paperwork falls on you to do in the clinic?
MEHTA: Most often, yes.
SOOD: REMS is done by the nurses. I have not done REMS myself. I have a nurse who is designated to do that, and she does more than that. It is not that big a hassle once you have it streamlined. We do it for my patients all the time; almost every one of my patients is on lenalidomide.
MODI: We do not have a pharmacist but have staff who work on this paperwork for us.
TORKA: Tafasitamab is an anti-CD19 monoclonal antibody that is FC-enhanced. It is a little bit genetically engineered, but it works like any other monoclonal antibody. [What are your thoughts on the data for tafasitamab plus lenalidomide]?
HERNANDEZ-ILIZALITURRI: When you look at the response rate of the patients based on cell of origin, the non–GCB DLBCL response was [approximately] 70%, which is much higher. Unfortunately, that was not the primary end point of the study, so it doesn’t get highlighted in the manuscript a lot.1 This is something to take notice of, especially when you are trying to identify the patients who most likely will respond to this regimen.
The other thing is looking at the dosing of lenalidomide….I think it’s very important to try to give as much as the patient can tolerate because, in contrast to multiple myeloma or chronic lymphocytic leukemia, low-dose lenalidomide barely has activity in DLBCL. I try not to go below 15 mg because I think you will lose activity in this kind of patient.
TORKA: These are good points. For the GCB and non–GCB points, which Dr Hernandez is alluding to, rituximab and lenalidomide had good activity in activated B-cell [aBC] DLBCL but it had barely any activity in GCB DLBCL; however, tafasitamab plus lenalidomide has blanket approval. In the manuscript, they don’t have the breakdown of GCB or non–GCB outcomes. So we reached out to the company to ask. The company shared the data showing that patients who have non–GCB or aBC DLBCL, because it is driven by the NF-κB pathway, respond better to lenalidomide, and the responses of tafasitamab plus lenalidomide are better in those patients.
In my practice, if I must choose between pola-BR and tafasitamab plus lenalidomide in a patient with non–GCB DLBCL, it is a no-brainer for me that I would go with tafasitamab plus lenalidomide at that point. I don’t think these data are published anywhere, but the response rates with tafasitamab plus lenalidomide are rather low in patients with GCB DLBCL. I would say they are probably 25% to 30%. In that case, it’s fair game whether you want to use tafasitamab plus lenalidomide or pola-BR.
SOOD: Does cell of origin evolve from one cell to another? After the initial biopsy, do you need to rebiopsy these patients?
HERNANDEZ-ILIZALITURRI: Normally the [cell of origin] doesn’t change the cell of origin. If they had GCB to begin with, they will have GCB to the end.
SOOD: There is no change of phenotype?
TORKA: We do recommend biopsy with each line of therapy because we want to confirm the diagnosis. Sometimes it’s obvious, but sometimes it’s not. It is important to test for that. If it is an older patient, I try to do bone marrow biopsies just to see what the bone marrow reserve is and what might be tolerated better for these patients. Both regimens are pretty myelosuppressive.
TORKA: Loncastuximab is less myelosuppressive, so that might be a consideration. It was just approved recently, so there is no familiarity with it. Is there any concern that you have with loncastuximab, or is it that it’s new, you are not familiar with it, and we don’t know what the niche might be?
STEINBERG: I have given it to 1 patient who had received polatuzumab in the past; they were inpatient, a little bit debilitated, and it would have taken a long time because we don’t have inpatient formulary for lenalidomide. We decided to use [loncastuximab]. When I shared the data with the patient and family, it sounded good: 48% ORR.2 The median time to response was [approximately] 2 months. It was quite an aggressive lymphoma. Overall, it seemed like a reasonable option short of doing palliative care at that point. So they were willing to try.
TORKA: Kudos that you were able to get it in the inpatient setting. That must have required a lot of convincing.
SOOD: We have an amazing pharmacist, and if the data are there, we can use it inpatient.
TORKA: I don’t think I can use it here at Roswell Park. They will not let me. But you bring up a good point…if you see time to first response, all the [later-line DLBCL] studies will tell you 2 months. That is because the first scan will be done in 2 months. So that is the official time to first response, but the clinical response is much faster.
HERNANDEZ-ILIZALITURRI: By the time they are getting third-line therapy…chances are they responded to treatment.
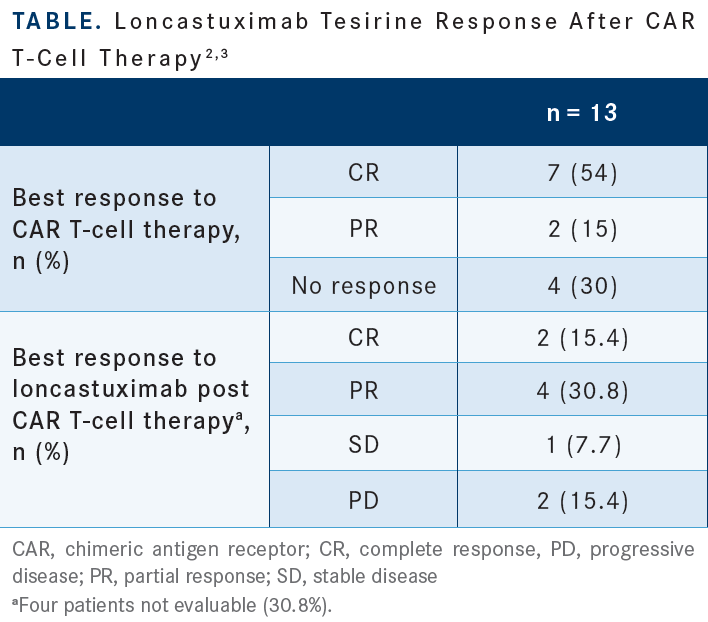
DAI: I think loncastuximab is an option for [older patients] who cannot tolerate aggressive treatments such as CAR T-cell therapy. The schedule is good; you do it once every 3 weeks and keep going. I would certainly choose it for the patient who is not a candidate for CAR T-cell therapy or transplant. There is concern that after you use it, it may knock off the CD19 and reduce the efficacy of future CAR T-cell therapy, even though the data show 15 patients who had this treatment and proceeded with CAR T-cell treatment and showed response [Table2,3]. I feel it is a treatment option for us in a select group of patients.
SOOD: I have not had a chance to use it yet. It seems tolerable and easier.
HERNANDEZ-ILIZALITURRI: We have used it at our institute. I think on the positive side it is very easy to administer. It is very well tolerated and some patients respond. Sometimes you can palliate them and give them a couple of more months with their families. But the duration of the response is short.
I think this is a good drug if you have a patient you want to get through the holidays without him or her being admitted to the hospital, trying to make the family happy, or trying to get them to some event. But it is not something that is going to give you long mileage. I think an important point you talked about is checking for CD19 expression prior to CAR T-cell therapy if a patient is to receive CD19-directed therapy. This is a situation in which a fresh biopsy is important because there is no accurate CD19 antibody test, so you rely on flow cytometry, and for that you need fresh, living cells. I think that is very important when you advise these patients and talk to the pathologist and tell them what you are looking for, because you are going to depend on flow cytometry for that.
TORKA: Dr Steinberg, you mentioned that you used it in an inpatient? What happened to that patient?
STEINBERG: The patient ended up passing away, just progression of disease.
TORKA: Did they get any benefit, even for a few weeks?
STEINBERG: Yes, initially the lactate dehydrogenase did decrease as a marker for response, but it was such a kinetically aggressive lymphoma and this was a last hurrah. Aside from palliative care, we decided to give it a try. I could see a role for it, maybe in less aggressive lymphomas, not those with say a Ki67 of 90%, but in DLBCL maybe with 60%, so not as aggressive. It’s just anecdotal, from my experience with 1 patient.
TORKA: None of us have used it all that much, so we must learn from each other’s experience.
STEINBERG: Maybe there will be studies giving it in combination in the future.
TORKA: Yes, many are coming.

MODI: It depends on the patient’s comorbidities, performance data, and the prior response. Let’s say the patient has bad neuropathy; polatuzumab wouldn’t be a great option, so we could use tafasitamab plus lenalidomide or loncastuximab.
TORKA: Absolutely. Comorbidities are something that must be considered. Lenalidomide is an older drug, which sometimes is a big problem because you have other options, so why spend so much time trying to get it.
REFERENCES
1. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
2. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/S1470-2045(21)00139-X
3. Caimi PF, Ardeshna KM, Reid E, et al. The antiCD19 antibody drug immunoconjugate loncastuximab achieves responses in DLBCL relapsing after antiCD19 CAR-T cell therapy. Clin Lymphoma Myeloma Leuk. 2022;22(5):e335-e339. doi:10.1016/j.clml.2021.11.005