Spigel Looks at Using Different Regimens Depending on PD-L1 Status in NSCLC
How an oncologist treats a patients with non–small cell lung cancer wit the available treatment options is largely dependent on the patient's PD-L1 status.
David Spigel, MD

David Spigel, MD, of Tennessee Oncology, discussed the case of a 59-year-old White man with non–small cell lung cancer with a a group of peers during a Targeted Oncology Case-Based Roundtable event.
Targeted OncologyTM: What are the potential treatment options for this patient?
SPIGEL: Right now, the NCCN [National Comprehensive Cancer Network] guidelines [for patients with non–small cell lung cancer (NSCLC) positive for PD-L1 expression (≥ 1%-49%) are quite complicated].1 There are a lot of options, but the preferred combination is carboplatin or cisplatin/pemetrexed [Alimta]/pembrolizumab [Keytruda].
We now have 3 PD-1 and PD-L1 inhibitors approved in the United States as monotherapy in the first-line setting: pembrolizumab, cemiplimab [Libtayo] used for squamous cancer, and atezolizumab [Tecentriq]. They’ve all shown a benefit to patients and were better than chemotherapy in high-expressing patients. Well, this patient doesn’t have a high expression. Pembrolizumab has been approved for patients expressing PD-L1 at 1% or higher, but pembrolizumab monotherapy is not the ideal choice for a patient with PD-L1 expression between 1% and 49%. I don’t think it works very well. If this patient had a PD-L1 expression of 100%, then yes, you could give him pembrolizumab. But because he’s symptomatic, you have no wiggle room. A lot of the pivotal studies show that [patients can drop off quickly] during the first 6 weeks. Chemotherapy seems to get them through those first 6 weeks. That’s why I love the preferred regimen outlined in the guidelines.
What is the evidence that supports using carboplatin or cisplatin, pemetrexed, and pembrolizumab?
The KEYNOTE-189 study [NCT02578680] was a pivotal trial. It was a very simple study with patients with advanced lung adenocarcinoma who were randomized, regardless of PD-L1 status, to receive either carboplatin or cisplatin plus pemetrexed/pembrolizumab or carboplatin or cisplatin/pemetrexed/placebo [for 4 cycles] followed by maintenance with pemetrexed plus pembrolizumab or pemetrexed alone.2
The take-home message [at the 23.1-month follow-up] from this large study of over 600 patients was that the group that got pembrolizumab with chemotherapy outperformed the group that got chemotherapy alone [median OS (overall survival) of 22.0 vs 10.7 months].3 [These results], considering the data are approaching 3 years, are pretty remarkable. Early on during the study, the 2 Kaplan-Meier curves were very close together, then they began to separate by the second or third month. They’ve analyzed the data in many different ways, and the bottom line is that it doesn’t matter what the PD-L1 expression level is.
There’s been some debate that patients with 0% PD-L1 expression don’t benefit from immunotherapy. However, the results from this study suggest they do benefit from chemotherapy plus immunotherapy with a HR of 0.52. This benefit is sustained as the PD-L1 expression changes. That’s why I think this is a good regimen because you can give it to everybody. It works, and it benefits them.
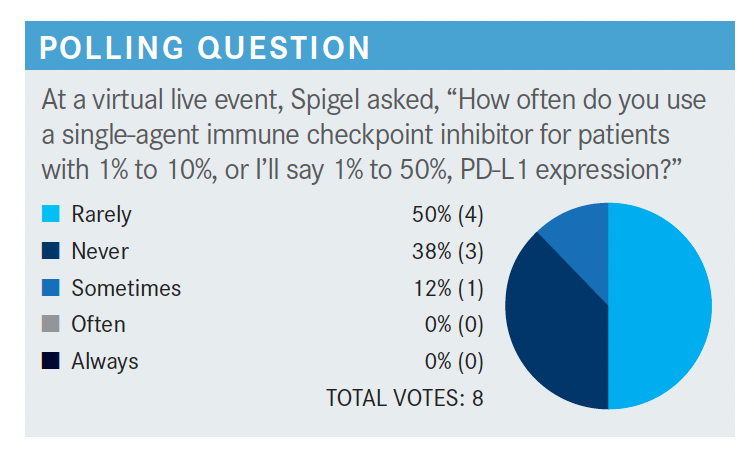
What are your thoughts on these poll results?
It looks like most people say “rarely.” I don’t think there’s a wrong answer here. If you’ve had good success [with using a single-agent immune checkpoint inhibitor for these patients], it makes sense to do so. I have not had good success in patients that are high expressors. In fact, I’ve tried to rescue patients with carboplatin plus pemetrexed after they get sick, and it’s too late, I can’t turn it around. Therefore, I tend to give patients the triplet regimen because I worry I can’t keep them from getting worse [if the monotherapy doesn’t work].
However, yesterday an [older], frail patient came in and I worried a little about giving him a triplet regimen. He had 100% PD-L1 expression and no symptoms, just sitting around and looking great, so I think this patient is a good candidate for pembrolizumab alone. Have I ever done it in a patient with less than 10% PD-L1 expression? Yes. There was an [older] woman who didn’t want chemotherapy, so I gave her pembrolizumab. It hasn’t worked, but pembrolizumab monotherapy is approved for patients who don’t want chemotherapy.
If the adenocarcinoma has a mixed histology, do you prefer to use pemetrexed or paclitaxel?
That’s a great question. Pemetrexed is a nonsquamous regimen. If there’s any squamous histology, you should use a taxane-based regimen. I prefer the nab-paclitaxel [nanoparticle albumin-bound paclitaxel; Abraxane] regimen, as we don’t have good data for gemcitabine [Gemzar], but you could also use paclitaxel. I don’t love nab-paclitaxel because of AEs [adverse events], such as alopecia and weight loss.4 I give it on days 1 and 8 and take day 15 off. I don’t give it 3 weeks in a row; that’s just too hard for patients. Sometimes I also give maintenance nab-paclitaxel. But yes, [you need to do that] for mixed histologies.
It’s not that pemetrexed doesn’t have activity in squamous carcinomas; it does. We [just] know taxanes have a little better track record in that group. Additionally, pemetrexed is worse than gemcitabine in patients with squamous carcinomas. Therefore, if it’s worse than gemcitabine in patients with squamous carcinomas, it’s also probably worse than a taxane.
What about other regimens? If you use other regimens, for which patient populations?
There are other possible regimens [for patients with nonsquamous NSCLC with PD-L1 expression between 1% and 49%]. [One option is carboplatin/paclitaxel/bevacizumab (Avastin)/atezolizumab].1 Some people are big fans of bevacizumab, and I used to use it a lot, then I just got away from it. I like paclitaxel, but I don’t like that it causes alopecia. Pemetrexed is so easy that I just can’t find a reason to give this regimen. However, some people think it’s the right regimen if the patient has an EGFR mutation followed by immunotherapy and chemotherapy. There were about 60 patients in a subset analysis that seemed to benefit from that regimen,5 but by no means does that make it the [go-to] EGFR regimen.
[Another option is carboplatin/nab-paclitaxel/atezolizumab].1 I talked about giving nab-paclitaxel, and there are data supporting the combination with atezolizumab.6 In my opinion, the atezolizumab data do not look as good as the pembrolizumab data. [There is also a nivolumab (Opdivo) plus ipilimumab (Yervoy) regimen alone or in combination with pemetrexed and platinum therapy. Finally, pembrolizumab monotherapy is available for certain patients.]1
What is the evidence for pembrolizumab alone for patients with NSCLC with PD-L1 expression between 1% and 49%?
The evidence for this came from the KEYNOTE-042 study [NCT02220894], which was a follow-up to the KEYNOTE-024 study [NCT02142738]. These trials were identical in design, except for 1 factor: PD-L1 expression. KEYNOTE- 024 was the pivotal trial that showed pembrolizumab is better than chemotherapy for patients with high PD-L1 expression.7 That’s the study that got pembrolizumab on the map. KEYNOTE-042 investigated whether pembrolizumab would have the same effect in patients with PD-L1 expression above 1%.8,9
The way the results from this trial have been interpreted concerns me. The OS curves [at various PD-L1 expression cutoffs] show that chemotherapy was better than immunotherapy for the first 6 months. This includes patients who have PD-L1 expression of 50% or higher.8,9 That is concerning because these data don’t replicate the KEYNOTE-024 data for high expressors. Why does this trial look so different? The curve for patients with PD-L1 expression between 20% and 49% shows less of a benefit with pembrolizumab than for high expressors. The curve including all patients [with expression] 1% or higher shows even less of a benefit.8,9 What everyone is reluctant to show with KEYNOTE-042 is that if you look at patients with PD-L1 expression between 1% and 49%, there really is no benefit to immunotherapy. However, it was approved by the FDA because there was a statistically significant benefit when all patients were included.10 But I just don’t think pembrolizumab has a role in the low expressors.
[An exploratory analysis of the 1% to 49% PD-L1 expression group shows that at 24 months, the OS is longer for the pembrolizumab group (13.4 months) than for the chemotherapy group (12.1 months). However, the curves show that up to 12 months, survival is higher in the chemotherapy group.]8,9 Maybe as you get past a year, immunotherapy starts to win, but who has a year to wait? So immunotherapy alone for patients with low PD-L1 expression does not get a thumbs-up, in my opinion.
What is the evidence for the use of atezolizumab for these patients?
The evidence for this comes from Genentech’s atezolizumab trial [NCT02366143]. It’s a complicated study. We didn’t open it in Tennessee because I didn’t think it was the best design. Basically, [the study had 3 arms comparing] carboplatin/paclitaxel combined with either atezolizumab alone, atezolizumab/bevacizumab, or bevacizumab alone.11
The OS analysis showed [a statistically significant and clinically meaningful benefit] for the quadruplet regimen vs the bevacizumab and chemotherapy regimen,5,11 so that’s an improvement. It’s interesting that the curves don’t show much of a difference until after 4 or 6 months. This seems to be consistent across all trials—it is what happens in the early period.
What is the evidence for the use of nivolumab/ ipilimumab for these patients?
[The evidence for this regimen comes from the CheckMate 227 study (NCT02477826)],12,13 which was a very interesting trial and created a lot of controversy. The study design was really complicated, but I’ll try to simplify it. Basically, the study compared nivolumab/ipilimumab vs chemotherapy alone or chemotherapy and nivolumab. There were a lot of different iterations, including the level of PD-L1 expression.
The results from this study were published in the New England Journal of Medicine and led to approval by the FDA.14,15 The take-home message from this study was that the nivolumab/ipilimumab group had a higher OS than the chemotherapy alone group [17.1 months vs 14.9 months, respectively].12 Are these results better than those from KEYNOTE-189? Is this regimen better than the atezolizumab regimen? There is no way to know, but it is better than chemotherapy. I have [several] patients on this regimen, and the best long-term survivors—folks who have no evidence of cancer—are those on nivolumab/ipilimumab. I don’t know whether that’s because that’s the way it is or whether I got lucky with those patients.
In this study, the PD-L1 expression level doesn’t seem to matter, but the big discussion with this trial is about the PD-L1 less than 1% group, [where the median OS for the nivolumab/ipilimumab group was also longer than that for the chemotherapy alone group (17.2 months vs 12.2 months, respectively)].12 This trial was not powered to get an FDA approval for just the patients with 0% PD-L1 expression, but there is a huge opportunity for it, as there is no other regimen for this patient group. It is interesting that nivolumab/ipilimumab looks very effective for this group that we worry about. Although, KEYNOTE-189 also showed that pembrolizumab is effective in that group, so there are times in the clinic when a patient has 0% PD-L1 expression and nivolumab/ipilimumab comes to mind.
I love this regimen for NSCLC, and it is probably even better to treat melanoma and kidney cancer. This regimen works best if you give ipilimumab at 1 mg/kg every 6 weeks, and nivolumab at 3 mg/kg every 3 weeks. OncoEMR has many possible combinations, which can be complicated, but that’s the regimen I like. This regimen has more toxicity. There’s no way around it. It has more toxicity than nivolumab or pembrolizumab, so you [must] keep that in mind. Rare but serious toxicities have been reported. I don’t mean to scare anybody, but one of our partners has a patient in the hospital right now who’s got near liver failure that we think is from an IO [immuno-oncology]/IO combination.
I’ve had 2 patients who developed type 1 diabetes following treatment, but I have more patients with CRs [complete remissions] who are long-term survivors—even some patients with small cell lung cancer. I’m a believer in the regimen, but you have to be prepared to deal with more serious toxicities than those experienced with a single IO agent. This can be tough to decide at the bedside or in the clinic. Am I prepared to deal with severe colitis, or some other severe complication? Those things can also happen with pembrolizumab or atezolizumab alone, but it’s rarer. [Nivolumab/ipilimumab had more TRSAEs (treatment-related serious adverse events) leading to discontinuation and treatment-related deaths than chemotherapy (24.5%, 18.1%, and 1.4% vs 13.9%, 9.1%, and 1.1%, respectively)].14 The most interesting trial was the CheckMate 9LA study [NCT03215706]. It compared traditional chemotherapy [for 4 cycles] vs nivolumab/ipilimumab together with 2 cycles of chemotherapy.16,17
This trial showed the best results right from the beginning [2-year follow-up, HR, 0.72]. Maybe this was because it included the chemotherapy for those first 2 cycles followed by nivolumab/ipilimumab. I like this regimen. You’re not keeping people on chemotherapy forever. Maintenance is done with pemetrexed, which is good and friendly, but nobody likes to stay on drugs forever. Hopefully nivolumab/ipilimumab gives the immunotherapy lift that is needed.
This regimen was effective in all groups, including patients with low [HR, 0.67; 95% CI, 0.51-0.88] and high [HR, 0.67; 95% CI, 0.46-0.97] PD-L1 expression.16 It’s kind of a one-size-fits-all recipe for squamous and nonsquamous NSCLC with low and high PD-L1 expression. This is the other no-brainer regimen. Is this better than that used in the KEYNOTE-189 study? We don’t know. Is this more toxic? Probably. On the other hand, this regimen has less chemotherapy than others, so there will be less toxicity from chemotherapy, but there will probably be more IO toxicity. However, the toxicity profile reported in this trial was reasonable. The rate for any-grade skin toxicity was 40%, which can be handled. The rate for any-grade gastrointestinal toxicity was 23%, with 6% being severe, which starts to get me a little nervous. [The rate for any-grade hepatic toxicity was 14%], so transaminitis that can be minor or severe.17,18
Both these regimens were approved by the FDA and are available now.15,19
What factors influence your decision-making for PD-L1–positive metastatic NSCLC? How do the newly reported data influence your decision-making?
I’ve used the CheckMate 9LA regimen twice since it was approved last June. It’s not that I dislike it, it’s just a weird regimen for me. The first question I ask is: Am I giving targeted therapy or not? Next-generation sequencing solves that question for me. Once I know I’m not giving targeted therapy, the next question is, am I giving immunotherapy by itself? I’m only doing it in the highest PD-L1 expressors who look great or the rare patient that refuses chemotherapy. For most of my patients, it’s chemotherapy plus IO. The next question is: What drugs am I going to use? I’m either going to use a pemetrexed-based regimen with pembrolizumab—that’s what I like—or I might give this CheckMate 9LA regimen and get more experience with it. The IO toxicity makes me a little bit nervous. It’s not that I wouldn’t do it, it just makes me a little bit nervous.
With the nivolumab/ipilimumab/chemotherapy combination, do you give ipilimumab for a year?
If I’m not mistaken, it’s 2 years of nivolumab/ipilimumab. This may be a little off subject, but at the American Society of Clinical Oncology 2021 Annual meeting, they showed the 7-year survival data for nivolumab/ipilimumab in melanoma. It was 49%, which is unbelievable.20
Does that mean nivolumab/ipilimumab is the best combination for all cancers? No, but there are some settings where you’re just going to give it and deal with the toxicity. Certainly, for metastatic melanoma, it’s a great option. For lung cancer, it looks pretty good. There are 2 phase 3 trials that show it is better than chemotherapy. I just don’t know that it’s that much better than carboplatin/pemetrexed/pembrolizumab, which I feel pretty comfortable with. I’ve used so much of that, and I know what to expect. There’s rarely surprises with that, so that’s the way I think through that.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 6. June 15, 2020. Accessed March 15, 2021. https://bit.ly/31aF6nS
2. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi:10.1056/NEJMoa1801005
3. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517. doi:10.1200/JCO.19.03136
4. Abraxane. Highlights of prescribing information. FDA. Accessed October 8, 2021. https://bit.ly/3aphS1K
5. Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi:10.1056/NEJMoa1716948
6. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi:10.1016/S1470-2045(19)30167-6
7. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi:10.1056/NEJMoa1606774
8. Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl 18). doi:10.1200/JCO.2018.36.18_suppl.LBA4
9. Mok TSK, Wu YL, Kudaba I, et al; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi:10.1016/S0140-6736(18)32409-7
10. FDA expands pembrolizumab indication for first-line treatment of NSCLC (TPS ≥1%). FDA. April 11, 2019. Accessed October 6, 2021. https://bit.ly/3uVtl2w
11. Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol. 2018;36(suppl 15):9002. doi:10.1200/JCO.2018.36.15_suppl.9002
12. Paz-Ares LG, Ciuleanu TE, Lee JS, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol. 2021;39(suppl 15):9016. doi:10.1200/JCO.2021.39.15_suppl.9016
13. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
14. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
15. FDA approves nivolumab plus ipilimumab and chemotherapy for first-line treatment of metastatic NSCLC. FDA. Updated May 27, 2020. Accessed December 21, 2020. https://bit.ly/35tnarl
16. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): Two-year update from CheckMate 9LA. J Clin Oncol. 2021;39(suppl 15):9000. doi:10.1200/JCO.2021.39.15_suppl.9000
17. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. Published correction appears in Lancet Oncol. 2021;22(3):e92.
18. Reck M, Ciuleanu TE, Cobo M, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38(suppl 15):9501. doi:10.1200/JCO.2020.38.15_suppl.9501
19. FDA approves nivolumab plus ipilimumab for first-line mNSCLC (PD-L1 tumor expression ≥1%). FDA. May 15, 2020. Accessed October 7, 2021. https://bit.ly/3uXPxsX
