Neparidze Covers Treatment Options Plus Use of Next-Generation Sequencing in NDMM
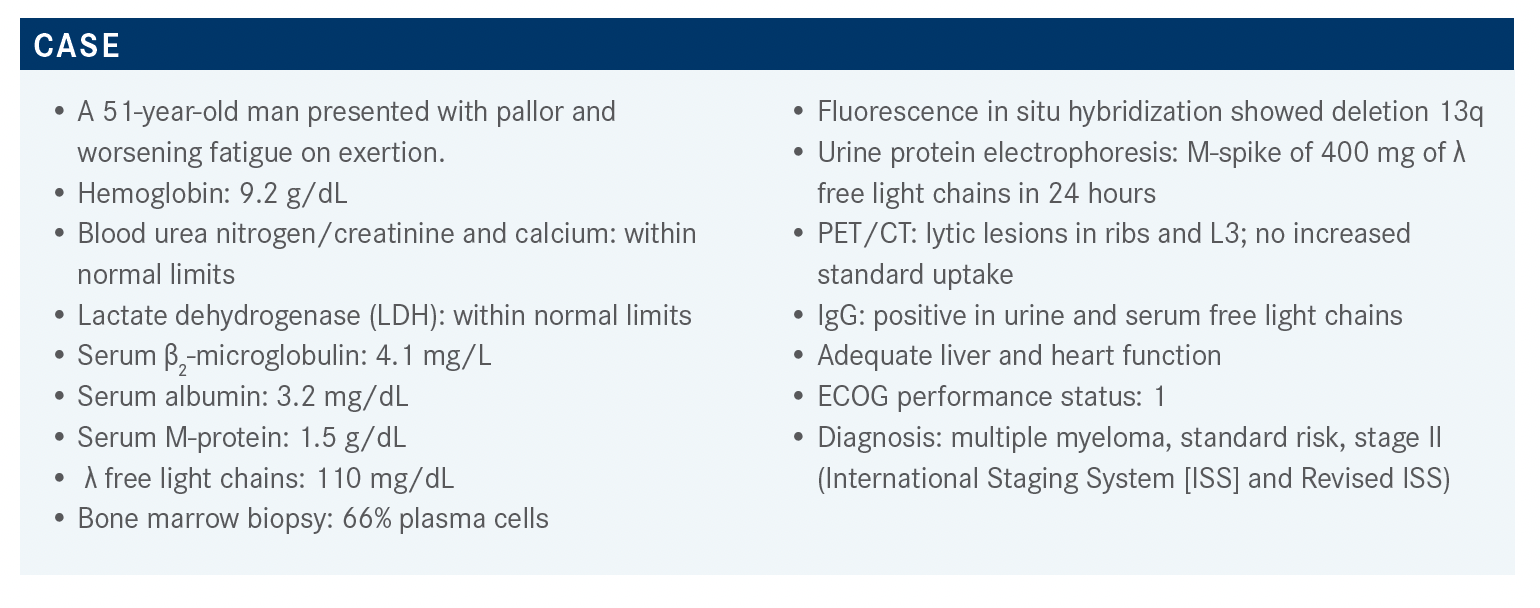
A 51-year-old man presented with pallor and worsening fatigue on exertion and was diagnosed with multiple myeloma, standard risk, stage II.
Natalia Neparidze, MD

During a Targeted OncologyTM Case-Based Roundtable event, Natalia Neparidze, MD, assistant professor of Internal Medicine (Hematology), research director, Myeloma Program at Yale School of Medicine/Yale Cancer Center in New Haven, CT, discussed he case of a 51-year-old man with newly-diagnosed multiple myeloma.
Targeted OncologyTM: What are the frontline therapy options for patients with newly diagnosed multiple myeloma (NDMM)?
NEPARIDZE: The NCCN [National Comprehensive Cancer Network] list of appropriate frontline regimens is continuing to grow but they still list based on the previous phase 1 and phase 3 data on bortezomib [Velcade], lenalidomide [Revlimid], [and] dexamethasone [VRd] induction that is still considered category 1.1 It is a reasonable regimen in some of the standard-risk patients with newly received diagnoses if they do not favor a whole-drug regimen [because of] whatever considerations.
The quadruplet regimen [daratumumab (Darzalex), bortezomib, lenalidomide, and dexamethasone (D-VRd)]—particularly for the transplant-eligible, standard-risk patient—is very reasonable. If this were my own family member, I would give them the most effective drugs in the frontline to achieve the deepest level of response.
Other regimens that are listed include carfilzomib [Kyprolis], lenalidomide, and dexamethasone [KRd], which is favored at certain institutions for high-risk patients based on the FORTE trial [NCT02203643] data. The care is evolving; down the line, we may have more regimens coming.
Does the regimen improve survival in patients with multiple myeloma?
It’s too early to judge that. There’s suggestion of progression-free survival [PFS] benefit. Overall survival [OS] is not there yet, and for the median follow-up it’s still too early to tell. We have detailed data on all the trials leading to the FDA label and approvals. Primarily, [the whole-drug regimen] translates into a deeper, higher proportion of minimal residual disease [MRD]–negative remission and, we know from other studies, a surrogate marker that [leads to] improved PFS and OS.
In 2021, what is the role of autologous stem cell transplant (ASCT) with so many new biologic drugs?
The IFM/DFCI [Intergroupe Francophone du Myelome/Dana-Farber Cancer Institute] randomized phase 3 trial [NCT01191060] took patients who were transplant eligible with NDMM and randomized them to VRd induction for 3 cycles plus ASCT collection, [followed by consolidation of cycles 4 to 8 of VRd]. The other arm received induction with [3 cycles of VRd] followed by high-dose melphalan and ASCT. In addition, they received consolidation of cycles 4 and 5 of VRd. Both arms continued maintenance lenalidomide.2 [At DFCI] in the United States this was done until progression of disease, but the European part of the study did the maintenance lenalidomide for up to 12 months. This was probably because of financial considerations.
The PFS of this study was significantly in favor of early ASCT. When we present the data to our patients and discuss the benefit of high-dose melphalan and ASCT, we always say it is the modality that may keep the patient in PFS the longest. It still is considered for patients in their 40s, 50s, and early 60s because it provides significant PFS benefit compared with the group with deferred transplant. However, the OS between the 2 groups was essentially the same.
In multiple myeloma…it’s very hard to demonstrate OS benefit when you have so many effective salvage agents. So, by textbook, in patients under 70—certainly under 65—I consider them for transplant early in the first remission because I believe this is a modality that will keep the patient in remission the longest, translating into PFS benefit, but also give the patient data on OS stating that benefit was not demonstrated.
An important point is that whether or not the patient received ASCT, if they achieved an MRD-negative status [then they] did better. So, the worst group was [individuals] who had deferred ASCT, got VRd alone, and had MRD-positive disease. It seems MRD negativity translates into much better PFS and OS across the board.3
[At the start of maintenance therapy], the median PFS was not reached [for the MRD-negative patients vs 29 months for the MRD-positive ones], which is significant. We still do [ASCT] at the Yale Cancer Center, and frontline, good performance status patients with standard-risk cytogenetics and newly received diagnoses will likely favor a quadruplet regimen like D-VRd followed by ASCT in remission [and] then maintenance therapy.
The FORTE trial, which is a European trial, was a very large, randomized study involving 477 patients. It had 3 arms [randomized 1:1:1] consisting of 4 cycles of KCd [carfilzomib, cyclophosphamide, dexamethasone] followed by ASCT, which was [the first arm]. The second arm consisted of 4 cycles of induction with KRd followed by ASCT. [The third arm] had 12 cycles of continued induction with KRd [KRd12] and no ASCT. Everybody underwent stem cell mobilization and collection, even if they deferred ASCT.
At the end of the phase of post transplant or consolidation, they were randomized [1:1] to either the lenalidomide [R] single agent as the standard-of-care agent or the carfilzomib plus lenalidomide [KR] combination maintenance.4 Both KRd12 and KRd plus ASCT did well. KCd was the least-favored regimen. After 3 years of median follow-up, the PFS curves showed superiority of KRd induction plus ASCT, followed by KRd12, and then the least favored— and certainly we would not use this regimen—is KCd. Standard[-risk] as well as high-risk patients and stage I as well as advanced stage II and III patients, and those with high LDH portending higher risk, fared equally well on KRd plus ASCT. That will be one of the good regimens that I would also perhaps consider among some of the advanced-stage and perhaps high-risk cytogenetic patients as a possible induction option.
By about 3 months of OS, a large proportion of patients were in CR [complete response] with a rather significant CR rate of 62% with KRd plus ASCT followed by KR maintenance. Continued maintenance for up to 2 years with KR added to some extent to the deepening of the response. High-risk patients such as those with high LDH fared equally well. The 3-year PFS rate [for the maintenance phase was 75% for the KR arm vs 66% for the R arm (HR, 0.63; 95% CI, 0.42-0.95; P = .026)].
How have some of the quadruplet regimens plus ASCT been evaluated in multiple myeloma?
The quadruplet regimens [are based on] some of the European data that I believe are not necessarily practice changing for the United States population, but the Cassiopeia trial [NCT02541383] had the first regimen that was published. It took transplant-eligible, NDMM patients and randomized them to either VTd [bortezomib, thalidomide (Thalomid), dexamethasone], which wouldn’t be the favored induction regimen in the United States, vs VTd plus daratumumab [D-VTd]. Everybody received 4 cycles of induction in both arms plus stem cell mobilization, conditioning, and ASCT in first remission. This was followed by 2 cycles of consolidation with either VTD or D-VTd [depending on the arm].5
The second randomization was to maintenance daratumumab for up to 2 years vs observation [until progression of disease]. Although this would not fly through Institutional Review Boards in the United States, this was the design of the study. These were patients who were under 65 [and] had good performance status; the primary end point was stringent CR [sCR].5
Cassiopeia demonstrated that the addition of the daratumumab increased the rate and the depth of the response. The sCR rate was much higher in the D-VTd arm as opposed to the VTd only, and overall sCR and CR rates favored the quadruplet regimen.
The same was true for MRD negativity, which is a surrogate marker that translated into much-improved PFS and OS. The subgroup analysis showed significant benefit in terms of PFS in the D-VTd arm. Note that in the study, not everybody was able to receive the subsequent daratumumab-based regimen in the second-line setting and beyond. The MRD negativity [rates after ASCT] were about 25% higher in the quadruplet regimen with D-VTd compared with VTd alone.
How often do patients get posttransplant consolidation as opposed to just starting them on maintenance therapy post transplant?
Because the FORTE trial and GRIFFIN trial [NCT02874742] have been published, we’re learning that what benefits [individuals] most is probably the continuation of the 2-drug or 3-drug regimen the longest. I personally have used a lot of MRD assessment in the posttransplant setting at day 100 and that helps me risk-stratify the individual in terms of the risk of recurrence. If they are MRD positive or have VGPR [very good partial response] or CR, I would be more in favor of continuation of the same regimen such as the quadruplet regimen I use in my practice, based on the published GRIFFIN study. If for some reason KRd was initially used in induction, we typically would go forward with the ASCT and at day 100 do the MRD assessment. Very frequently I would resume the KRd consolidation and replicate what was done in the FORTE trial, which is 8 total cycles of KRd induction and then ideally maintain those patients with KR.
The GRIFFIN study was published in the journal Blood in July 2020,6 and then subsequent updates on MRD analysis were presented later at the ASH [American Society of Hematology] conference in December 2020.7 It was a phase 2 randomized study with about 100 patients in each arm. Patients had transplant-eligible NDMM with good performance status and preserved renal function. The randomization was 1:1 to receive either the all-standard VRd regimen vs the D-VRd regimen. The standard dose of lenalidomide was used. Bortezomib dosing in the initial cycles was on days 1, 4, 8, and 11.6 In real practice, we sometimes modify that depending on patient tolerability and switch to the weekly dosing.
All patients received 4 cycles of induction with these respective regimens; then they were mobilized, collected, and received high-dose melphalan plus ASCT. Almost all patients received ASCT in the first remission. This was followed by 2 more cycles of consolidation therapy with either VRd or D-VRd followed by maintenance in their corresponding arms with either RD for up to 2 years or the standard-of-care maintenance with R alone.
The primary end point was sCR and the secondary end points were overall clinical responses, VGPR plus CR, as well as MRD, as assessed by next-generation sequencing [NGS].
Many of the IFM and European studies used flow cytometry, multiparameter, or next-generation flow [NGF] cytometry for their MRD assessment, which, depending on the lab, may translate into a sensitivity of 10–4 or 10–5 in the most modern 8-colorant laser, or 7-color or 8-color laser technology. The NGS offers a slightly deeper sensitivity of 10–5 or even a higher sensitivity of up to 10–6. The median age of patients was 60. A small proportion of them had stage III disease and about 15% had high-risk disease in this study.
What were the results of the GRIFFIN trial?
The primary finding of the study is that the D-VRd regimen achieves much earlier, deeper responses in MRD-negative rates in these patients. The CR rates are significantly higher. At the time of maintenance, over 60% of patients were in sCR as opposed to some 30% to 40% of patients on just VRd.7
In a majority of the subsets of patients, the D-VRd was favored, but [the benefit] was not as robust among high-risk patients [such as those with high-risk cytogenetics and stage III disease]. The high-risk cytogenetic patients represented only about 15% of the study population. All subsets of patients [including those with stage III disease] and high-risk cytogenetics were able to achieve MRD negativity, which was predominantly a favorable outcome of the D-VRd group.
So far, the data are not mature enough to clearly demonstrate OS benefit. There is some suggestion of durability of PFS and an OS benefit with the quadruplet regimen, but we need to wait and see further follow-up data on this study.
The quadruplet regimen for patients who are transplant eligible translates into a deeper, earlier response that appears to be durable. As evidenced by other studies, MRD-negative responses translate into better PFS and OS outcomes. Therefore, the D-VRd regimen enters our frontline therapy for many transplant-eligible patients.
For expected adverse events [AEs] for D-VRd, hematologic AEs are the primary toxicity. In real practice, they’re all manageable and amenable to close monitoring and adequate supportive care. Neutropenia of any grade, as well as some lymphopenia and anemia, and grade 3 and 4 neutropenia are seen more commonly with the quadruplet regimen.6,7
However, other general AEs are largely comparable, perhaps except for upper respiratory tract infections [URTIs], which was 62% [for D-VRd] vs 45% [for VRd]. However, none were grade 3 or 4 and the infusion reactions are primarily seen with quadruplet regimens. The toxicities are primarily neutropenia, cytopenia, URTIs, and infusion reactions, which are quite manageable in real practice.
Can you discuss how these 4 trials relate to each other for this population?
To summarize and synthesize all 4 studies, the IFM and [FORTE trials] were on early vs late transplant, Cassiopeia was on D-VTd quadruplet regimen, and the GRIFFIN study was on D-VRd quadruplet regimen. These are all highly effective regimens resulting in VGPR rates in the 70% to 80% range, probably 90% in the GRIFFIN study. In most of the recent studies with quadruplet regimens, as well as the FORTE trial, the median PFS is not even reached.2-7
What’s encouraging across the board is that all these regimens—particularly the GRIFFIN study, which used NGS as its primary way of assessing MRD—[had MRD negativity rates in] the order of 60%. The IFM and FORTE study primarily used NGF as the MRD assay, but in the FORTE trial they used the 7-color or 8-color laser, which translates into a sensitivity of 10–5. So, one could say this is relatively comparable across the board. KRd, D-VTd in the right geographical setting, or D-VRd are considered reasonable frontline regimens.2-7
There are additional quadruplet regimens coming in terms of evolving clinical trials employing daratumumab or similar monoclonal anti-CD38 monoclonal antibody backbone combinations with quadruplet regimens. The Perseus trial [NCT03710603] is a much larger study going on in Europe and around the globe with subcutaneous daratumumab, as well as the GMMG HD7 trial [NCT03617731], which is using isatuximab [Sarclisa] plus VRd vs VRd alone. So, there are more data to come.
The unmet needs in multiple myeloma, both in the frontline and late relapse/refractory settings, are high-risk patients who don’t enjoy such a long OS and PFS interval even with these interventions and frontline ASCT. This is something we as a community need to address, [as well as] develop trials, and use something entirely different and potentially other combinations such as the anti-CD38 monoclonal antibody as a backbone, adding other drugs, potentially bringing the most effective biologics to the earlier lines of therapy.
Do patients ever ask what the point of transplant is if they go back to the same regimen they were on before?
In the IFM study, all patients who deferred transplant got 8 cycles of therapy, but the transplant still brought PFS benefit. I would argue that transplant provides some degree of cell depletion and purging for any residual disease. But I agree, it’s not an ideal therapy and many patients might question this. Many of our patients may be transplant eligible but choose to defer transplant in the first CR. It’s a matter of perspective.
Our director of hematologic malignancies [at Yale Cancer Center], Steven Gore, MD, kept asking why we still do high-dose melphalan without curing anybody. But looking at the PFS, which is a reasonable end point for multiple myeloma, it has benefit. PFS may be a reasonable end point in indolent diseases such as multiple myeloma or other cancers across oncology. It is a significant end point, and high-dose melphalan is still a modality that provides the longest PFS even with modern triplet regimens. We give the option to patients and it’s a patient-centered decision.
ASCT is still considered a standard-of-care regimen to this day, and certainly in patients under 65, but it comes at the expense of significant toxicities up front. The morbidity rate is reduced and it is the modality that delays time to progression the longest compared with other therapies.
If one goes to Boston and has a consultation with Paul Richardson, MD, [DFCI], they will likely be persuaded to defer the ASCT. But again, I believe it has a significant benefit for patients who are much younger, in their 40s and 50s. You can tell patients that both groups of patients with early or deferred transplant would have the same longevity, but the group that got ASCT in first remission stays in remission significantly longer and that’s the benefit.
Ultimately, I would suggest everybody have a consultation with a stem cell specialist, certainly for patients under 70 or 65 with good performance status, to do the assessment. At the very least, every patient should have a stem cell collection. Before they get more than 4 to 5 cycles of lenalidomide-based regimen, consider stem cell collection. Even if they choose to defer the transplant, that will be saved in the blood bank as the next intervention because you could use them at the second CR.
What are your thoughts on using quadruplet regimens in patients with NDMM?
In the face of the recent GRIFFIN data, I would say that we have strong data on D-VRd. The Perseus study was still open in a few United States institutions, but I think it predominantly [is in] most centers in Europe. We do not have either of these trials available. But even if we did…I always encourage patient participation on clinical trials simply because GRIFFIN was a phase 2 randomized trial, not a phase 3 with very strong data. A larger study will shed more light on the overall benefit, particularly in the high-risk subgroup of patients. But I feel very comfortable just recommending D-VRd, particularly in standard-risk patients. I think it’s a highly effective regimen. After induction, patients can decide about what they favor and whether they would like to have a stem cell collection vs proceeding with high-dose melphalan in first remission. It’s really a multidisciplinary decision and I would encourage everybody to make a referral to a transplant center—and at the very least, young patients should have a stem cell collection if not an immediate transplant.
Given that isatuximab is moving up front, how does one choose the antibody backbone on which to put patients?
There are absolutely no data comparing these 2 monoclonal antibodies and never will be, so it’s hard to say. There is complete lack of data, and even if I gave an answer it’s not evidence based. Would I favor isatuximab-based frontline induction or daratumumab-based ones? I don’t know.
There are more data coming with even more potent combinations. D-KRd data from the pilot study at Memorial Sloan Kettering Cancer Center, which was highlighted within our journal club presentation, show amazing response rates of 100% and amazing MRD-negative rates. A similar study of isatuximab-KRd is being done and others at DFCI are continuing to enroll. So, there are more data coming.
There’s no answer as to which antibody backbone is better. We do know from general biologic pharmacokinetics-pharmacodynamics and receptor affinity data that daratumumab and isatuximab are quite comparable in terms of their mechanisms of action and cell-mediated and complement-mediated apoptosis. Some say that perhaps isatuximab has more direct apoptosis, but it has never been compared in a head-to-head manner and probably is never going to be.
We do know that all the isatuximab studies—like isatuximab, pomalidomide [Pomalyst], dexamethasone and isatuximab, carfilzomib, dexamethasone—generally excluded patients who were refractory to daratumumab. We don’t know whether there is still a role for isatuximab. Take the chronic lymphocytic leukemia experience, for instance. You do have anti-CD20 monoclonal antibodies that are second-generation type 2 antibodies, like obinutuzumab [Gazyva] and ofatumumab [Arzerra], which still have a role. Could there be a similar role for isatuximab? That remains to be determined.
In the [December 2020] ASH, there was a case series from Robert Z. Orlowski, MD, PhD, stating that in his practice he had a case series of about 10 patients or so, where he claimed that even with prior daratumumab refractoriness, when they were treated with isatuximab combinations, patients achieved responses. But there are very scant case series[–based] and case reports–based data.
In my own practice, when [patients] come in the late refractory setting, [with the] isatuximab combination, even with prior daratumumab failure, I do not see these responses generally. But again, there is a complete lack of data.
Why is there such a big difference of MRD negativity rates in the VRd arms of the IFM study and the GRIFFIN study?
It’s a somewhat different comparison because the MRD assay in the IFM was flow cytometry–based vs the GRIFFIN study, which used NGS for MRD assessment. In the subset analysis in the IFM study, they also did the MRD assessment by NGS, which has higher sensitivity.
Some say that when they did it on the subset of patients, the MRD negativity was only 30%, but that’s not in the original New England Journal of Medicine [report] comparing the early vs late transplant in the IFM study. It’s somewhat difficult to compare because the MRD negativity was done by flow cytometry assay, and in this particular study their flow cytometry assay had a 10–4 sensitivity vs in the majority of these studies, and specifically in the GRIFFIN trial, where NGS has a sensitivity of 10–5 or even higher.
Is NGS testing the preferred method of assessing MRD negativity?
Depending on the availability of the test, in our practice we frequently use both [NGS and flow cytometry] at the end of either induction or posttransplant day 100, because this is where the data are. At Yale Cancer Center, the flow cytometry that we do for multiple myeloma for posttransplant assessment is already a high-sensitivity multiparameter flow. It essentially completely resembles the original San Miguel 8-color flow with 8-color laser, which is again multiple 8-color stains. In our own lab, it has a sensitivity of 10–5. So, in assays, they capture approximately 1 million events and look at the clone.
In transplant-eligible patients at posttransplant day 100, when I do the response assessment, we do send a flow cytometry automatically and Yale’s own lab is doing that. But I do often use the NGS MRD as a higher sensitivity assay. At one of the past International Myeloma Working Group meetings, there was a discussion of this kind and counterargument…debating what’s most valid.
What the community is going to be distilled to eventually is that we are going to use MRD and it’s extremely prognostic. It can be used eventually as a surrogate marker of future prognoses in terms of determining PFS, OS, and risk stratification. It helps me in risk stratification of patients. But it is not a standard of care to perform it for everybody and change therapy based on its result, although it does guide us in the posttransplant setting to risk-stratify patients.
Eventually, I think what the community is going to move forward with is to really believe in the validity of MRD, especially a sustained MRD, which is what we showed when we look at the maintenance in the GRIFFIN and FORTE KRd data. What’s most significant in terms of prognostic assessment is this so-called sustained MRD. Whether it’s done by flow, high-sensitivity flow with 10–5 sensitivity, or the NGS, I think either one of them can translate into a significant PFS and OS benefit, especially if it’s a sustained MRD measure like after 12 months.
Again, this is still an area of active research. But for now, as part of standard of care, you can certainly apply both flow cytometry MRD as well as NGS MRD at the end of induction or posttransplant day 100 for risk-stratifying the patient, and one could consider repeating it at a 12-month interval, because that’s what was done in the studies.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 1.2022. Accessed October 6, 2021. https://bit.ly/3fa9Yx5
2. Attal M, Lauwers-Cances V, Hulin C, et al; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
3. Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. doi:10.1182/blood-2018-06-858613
4. Gay F, Musto P, Scalabrini DR, et al. Survival analysis of newly diagnosed transplant-eligible multiple myeloma patients in the randomized Forte trial. Blood. 2020;136(suppl 1):35-37. doi:10.1182/blood-2020-136907
5. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. doi:10.1016/S0140-6736(19)31240-1
6. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
7. Kaufman JL, Laubach JP, Sborov D, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of Griffin after 12 months of maintenance therapy. Blood. 2020;136(suppl 1):45-46. doi:10.1182/blood-2020-137109

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More