Roundtable Discussion: Johnson Reviews Molecular Testing and Options After Osimertinib in NSCLC
During a Targeted Oncology Case-Based Roundtable event, Melissa L. Johnson, MD, director, discussed the case of 66-year-old man with non–small cell lung cancer.
Melissa L. Johnson, MD

During a Targeted Oncology Case-Based Roundtable event, Melissa L. Johnson, MD, director, Lung Cancer Research, Sarah Cannon and Tennessee Oncology in Nashville, TN, discussed the case of 66-year-old man with non–small cell lung cancer.
JOHNSON: How often do you receive insufficient tissue? That is a reason, potentially, that you might not wait for molecular profiling if you knew you had a poor sample. Some other questions just along that line of thinking: What should the medical oncologists be doing to help ensure sufficient tissue is collected and that it’s used efficiently? Do you expressly call a pathologist to communicate what you want? Or do you expressly call the interventional radiologist or pulmonologist? All of that takes time.
HEMPHILL: Yes, there are times I’ve talked to the pathologist directly, as far as sequencing. Just because if you have a patient who is a never smoker, female, with adenocarcinoma vs [the] exact opposite, a patient who is male, uses tobacco, started smoking when [he was] 8 years old, with squamous cell, you’re thinking of different things. In the first scenario, if we think there’s going to be a limited sample, then I’ll ask them to do the [immediate test] on the EGFR pathway, and then whatever tissue is left over, trickle in the rest of [the testing].…I tend to sway toward EGFR [inhibitors] pretty heavily first and then go from there. Simultaneously, I get a liquid tumor biopsy as well.
JOHNSON: Do your pathologists wait to hear from you? Is there a reflex pathway that happens if you don’t say anything?
HEMPHILL: It depends on the day of the week. I think I’d get the NTRK [test results back] before I find out it’s cancer. It’s amazing, and everything else is way later.
JOHNSON: You should be able to get EGFR and ALK [results] that quickly as well.
KANDRA: In our institute for the past year and a half, the pathologists have stopped sending any molecular testing. They just let us order the next-generation sequencing [NGS] of choice. The NGS panel also does the PD-L1— pretty much everything. We just felt that some of these companies, like Caris [Life Sciences] or FoundationOne, know how much sample to use for which and what. If you send it to 2 separate locations, sometimes we have found tissue is not being well used.
JOHNSON: Do they do immunohistochemistry [IHC] for adenocarcinoma vs squamous and then stop?
KANDRA: If you send to Caris, or I think even for Foundation, they are doing the PD-L1 first by IHC and then using the rest of the sample for their NGS.
JOHNSON: Everybody has their own algorithm. Increasingly at [TriStar] Centennial Medical Center, we have started asking the pathologists to stop; get me a diagnosis of lung cancer and then stop, and I’ll take care of the rest. But the challenge with that is that nothing will happen, then, until they’re in clinic to see you, and you can start the testing process.
I know some of you will use liquid biopsy as well. Are you getting it the first time that patients come to see you in the clinic, or are you able to get it in the hospital?
KANDRA: We have to wait for the hospital discharge before [we] can send to Caris or FoundationOne.
CARR: I’ve never ordered in the hospital. Dr Ma, you see probably more people…than I do. Have you ever ordered it in the hospital?
MA: No, I have not. I have worried about double ordering. I get patients upset later on if I do 2 [molecular] profiling tests, and they get a big bill. I don’t usually do that in hospital. I will usually just send for our NGS now—the in-house testing. Within Tennessee Oncology, there’s a trick. You can preorder it. I have new patients; I order all the testing way before they see me. You can [submit the prerequest to] the pathology clerk way ahead of time, and they will work on it. You don’t have to wait for the patients’ first appointment.
JOHNSON: You have to wait until they’re discharged from the hospital to do that?
MA: I will request it because a lot of times, they can get the block [of time]. That’s the main thing that takes forever is to get the block. So they can go ahead and get that started. As soon as I see the records and I know I need it, I’ll go in oncology, prerequest it, and the pathology clerk will process it and request the block. So I will rely on [that date] to know what date to start the testing because the result will take 2 to 3 weeks, sometimes, to come out. For any in-house, in-hospital testing, I haven’t ordered Guardant [Health, Inc] or anything for the inpatient setting, no.
JOHNSON: I don’t think you’d be able to, honestly, because the whole problem with the 14-day rule is that if the tissue is acquired during the hospital stay and you order the testing while the patient’s still in the hospital, then the NGS testing gets include

d in the diagnosis-related group payment for the hospital stay. Even at Centennial, we’ve tried to get the Guardant 360 test on the formulary, and it’s been tricky to do. The best I’ve been able to do is get it when they come to see me in the clinic. I haven’t figured out how to game it any more than that. But Dr Ma’s idea is a good one; at least you can order it, and the pathologists can get the block before you see the patient.
That underscores the real issue, to me, which is just how difficult the testing is if your patients are coming from different hospitals—if you’re not an ivory tower [with] in-house NGS for DNA and RNA. I think that’s the problem that needs to be fixed because oncologists, for the most part, understand how important the testing is.
JOHNSON: I’m not surprised that the patient’s molecular profiling came back with an EGFR exon 19 deletion. I think it is fascinating, and I don’t understand why, that as much as 70% of patients with EGFR-mutated lung cancer express PD-L1 at 1% and higher—why that would be when they don’t do as well with a single EGFR tyrosine kinase inhibitor [TKI]. There’s some science to suggest that it’s a mechanism of resistance to EGFR TKI therapy. But why it would be upregulated from the beginning, I don’t know, but it is interesting. So you’re always going to have this conflict in these patients that they [have] some amount of PD-L1 expression and an EGFR mutation.
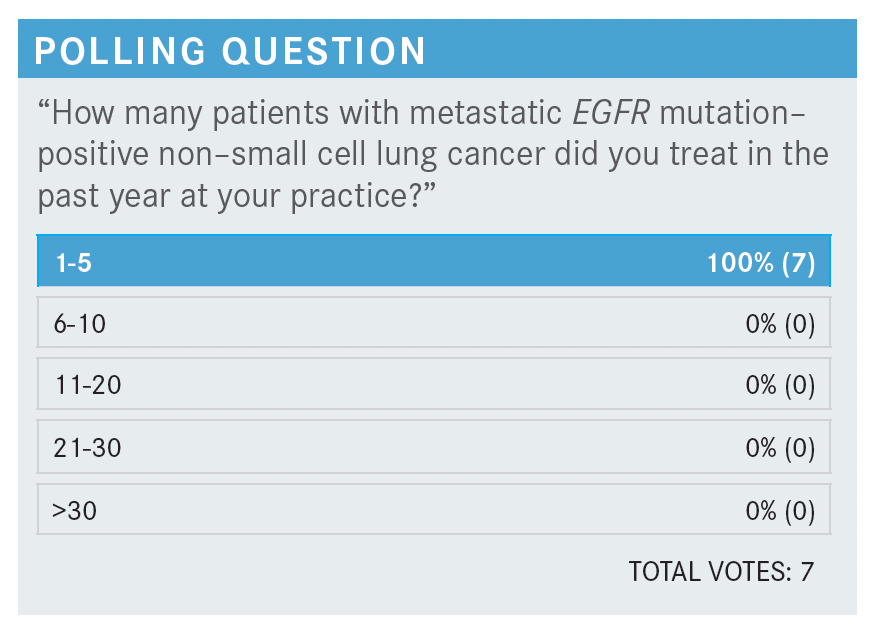
JOHNSON: Everybody’s saying about 1 to 5, and I think that’s probably about right. It’s supposedly 10% to 15% of White patients with non-small cell lung cancer in the United States and higher in Asia. But it doesn’t seem quite as high here in Tennessee, for whatever reason, and I would imagine Kentucky is like that as well.
This patient has a brain metastasis, so we’re going to be tempted to do some sort of treatment directed at that. Remember that it was 10 mm. Would any of you be tempted to start osimertinib [Tagrisso] and waive radiation, or would we all do radiation?
HEMPHILL: I think if there’s no edema and it’s 1 cm and asymptomatic, then yes, you could do that. It crosses the blood-brain barrier. I think it would be interesting to see, even from a prognostic standpoint, if there is shrinkage, just watching closely. I think that’s absolutely valid.
CARR: Yes, I’ve done that at least once before in somebody with 2 small brain metastases that may have been about a centimeter in size. But if there’s no vasogenic edema, no symptoms, then yes, I would agree with Dr Hemphill. I think I would probably go ahead and start the osimertinib. We know that the response rate in the brain’s pretty good. Repeat an MRI in 6 weeks or if they had any new symptoms, and then see how things are looking.
JOHNSON: We do find ourselves having this problem: We have started the immunotherapy without all of the data, and then, whether it’s 10 days later or 10 months later, when you finally get some decent molecular profiling, you find the EGFR mutation. It does happen.
MA: I’m not sure how convinced I am. It’s not that many cases [with EGFR in this trial (n = 11)], and we use immune therapy so often now. I can run into 5 patients straight who had complications, and then, suddenly, nobody had anything wrong. Everything’s fine, no problem for a long time, then all hell breaks loose. I feel [the number in the study is] so small. I don’t know [if] I hurt patients who were EGFR-mutation positive. Even if they had PD-L1 expression, they tend to be very low expressors. This patient in question, in the talk, has 90% expression. Wouldn’t that be a very unusual case for a patient with an EGFR mutation to have that high PD-L1 expression?
JOHNSON: I don’t think that’s true. I think patients with EGFR mutations can have very high PD-L1.
It just doesn’t seem to have the predictive power of the EGFR wild type…just like we know PD-L1 isn’t a perfect biomarker in our wild-type patients. Sometimes you have a patient that’s PD-L1 high, and then they don’t do well on pembrolizumab. I think there must be some other inhibitory mechanism or something else that’s holding back the immune system from acting the way we would expect with pembrolizumab; [that is] how I understand it.
Your point is well taken, though. It’s a small data set. It was supposed to be 25 patients, and they stopped enrollment at 11. So to make a blanket statement that no patient with EGFR-positive disease will benefit from immunotherapy feels wrong based on 11 patients. We have patients in the drug development unit who are on some of the first PD-L1 inhibitors who have EGFR mutations. The difference for them, though, is that they were heavily pretreated. They had all the TKIs plus every chemotherapy. They had really indolent disease. And so you wonder for a patient like that [whether] they accumulated new antigen burden in a different way over time, with cytotoxic chemotherapy, potentially.
When I look at these data, I take [them] with a grain of salt to say: It doesn’t mean that EGFR-positive patients should go to hospice without immune therapy, but rather it shouldn’t be the first or the second thing that you do.
MA: For this patient, if they progress on osimertinib [Tagrisso], after that, would you go to chemotherapy and not consider immune therapy in the second line?
JOHNSON: I typically don’t use immune therapy with my chemotherapy for patients with EGFR-positive disease, or ALK rearrangements if I get there. Now, we do have a trial sponsored by Arcus [Biosciences, Inc; NCT03846310] for patients that progress on osimertinib. They can get chemotherapy and Arcus’ PD-1 and/or their A2aR [etrumadenant; adenosine A2a receptor antagonist] because A2aR is also another mechanism that we see in EGFR-positive tumors. The idea is that maybe those 2 checkpoints together might work differently—maybe that gets around the inhibition that we don’t understand.
So there are ways to do it. The other data set that we have is IMpower150 [NCT02366143]. That’s the only chemoimmunotherapy regimen that included EGFR- and ALK-positive patients. Those patients got carboplatin or paclitaxel/bevacizumab [Avastin]/atezolizumab [Tecentriq], and while the primary end point was progression-free and overall survival in the wild-type population, the twist of art that has happened is there was more benefit for the EGFR-positive population in that trial. The FDA approved it in the wild-type patients, but you can get this regimen for your EGFR-positive patients, post osimertinib.
HEMPHILL: In 2021, would you not do a ramucirumab [Cyramza]/erlotinib [Tarceva] combination as your second line?
JOHNSON: That would probably be my third line. I would do docetaxel/ramucirumab [in the] third line after a platinum/pemetrexed [Alimta]–based regimen. I haven’t used the IMpower150 regimen much, to be honest. I just go to carboplatin/pemetrexed. I have added pembrolizumab before; I haven’t had toxicity. But after the TKI—the other way around—I’ve had it where I had a patient who had chemoimmunotherapy because I didn’t have the molecular profiling back, and then I gave him a rip-roaring hepatitis. I had to stop the drug, let him cool off, give him steroids. One technique I learned from Leora Horn, MD, MSc, before she left Vanderbilt-Ingram Cancer Center, was if you find yourself in that position, having given chemoimmunotherapy and finding the EGFR mutation, to start at half-dose osimertinib, and then I’ve done that subsequently. If there’s no toxicity, then increase [the dosage] over time.
JOHNSON: I’m curious what the next step would be for people. Do you order a repeat biopsy or liquid biopsy for mutation testing?
EZE: For most of my patients once they progress these days, I generally consider repeat biopsy. So, yes, definitely I would consider that in this case.
JOHNSON: Do you do repeat tissue biopsy, or are you using plasma, liquid based?
EZE: Both if I can, but if I can’t, I would send a liquid biopsy out.
JOHNSON: What are your therapeutic options at this point? Do you continue the TKI and rely on local therapy for the brain metastasis and the liver metastases? Would you start a checkpoint inhibitor second line? Would you do chemotherapy and bevacizumab, monoclonal antibody targeting angiogenesis?
EZE: I’ll probably do [carboplatin/pemetrexed] in this patient. If I remember correctly, he had 90% expression. So based on the data, this is not someone you want to give immunotherapy, at least not in second line. I would probably go ahead and give him combination platinum-based chemotherapy at this point.
HEMPHILL: If they [are] oligometastatic, I think I might have tried to nurse the treatment a little bit longer and do locally directed therapy if it’s just 1 isolated brain metastasis or 1 liver metastasis because, in general, it’s so well tolerated. You’re also still betting that hopefully, you buy another 3 to 6 months and there will be new options, maybe, at that point. But with 3, I think it becomes a little more difficult to make that argument. So I would [use] chemotherapy, too.
JOHNSON: Yes, I agree. This is a huge unmet need, and we have a couple trials that are opening right now; [some by] Daiichi Sankyo. [An antibody-drug conjugate] for patients with actionable genomic alterations and also a HER3 antibody-drug conjugate reported on at the American Society of Clinical Oncology 2021 Annual Meeting for patients with resistance to osimertinib. The trick for both is that patients had to have chemotherapy before they can go on those trials.
REFERENCE
Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138-1145. doi:10.1016/j.jtho.2018.03.035
