Hall Breaks Down Treatment Sequencing for Patients With Medullary Thyroid Cancer
Twelve months following a thyroidectomy with bilateral central neck dissection and later declining systemic therapy, a 58-year-old man with medullary thyroid cancer was no longer asymptomatic.
Richard D. Hall, MD, MS

During a Targeted Oncology Case-Based Roundtable event, Richard D. Hall, MD, MS, associate professor, University of Virginia School of Medicine, UVA Health in Charlottesville, VA, discussed the case of 58-year-old man with medullary thyroid cancer.
Targeted OncologyTM: Would you recommend systemic therapy for this patient? Would you order any mutational testing at this point?
HALL: Although MTC comprises only a small percentage of all thyroid cancers, 60% to 90% of cases will have RET gene alterations, [frequently will have] mutations, and sometimes [will just have] fusions.1
So if the patient has MTC, you should perform RET gene mutation testing and even more advanced next generation sequencing [NGS] testing as well. Some papillary thyroid cancers also have RET mutations. Most patients with MTC will have RET alterations, so the question is whether these are germline or sporadic, and we know this patient was germline negative, so looking for common mutations is important.
Do you normally order an NGS panel, or is there a way to order just RET?
I think that most [clinicians] order NGS panels because it’s more comprehensive. In other thyroid cancers—particularly in papillary thyroid cancer—you can see BRAF mutations. There are other gene changes that you can see in other types of thyroid cancer, but I don’t know if anyone’s ever ordered a test for RET mutations only. In other cancers, such as lung cancer, you often order [fluorescence in situ hybridization testing] or another panel to look for fusions, but in MTC it’s usually point mutations.
Which therapy would you be most likely to recommend?
According to the most recent NCCN [National Comprehensive Cancer Network] guidelines, vandetanib [Caprelsa] and cabozantinib [Cabometyx] have category 1 recommendations, meaning that there are randomized control trial data that justify giving these agents. Additionally, selpercatinib [Retevmo] and pralsetinib [Gavreto] are recommended for patients with RET mutations.2
What is the evidence for the use of vandetanib?
The ZETA trial [NCT00410761] was a phase 3 study that compared vandetanib with placebo. The study enrolled 331 patients randomized 1:1. The overall response rate [ORR] was 45% [P < .001] for vandetanib with a good disease control rate [87%; P = .001]. There was also a significant reduction in calcitonin at 69% [P < .001] and carcinoembryonic antigen at 52% [P < .001].
The HR for PFS [progression-free survival] was 0.46 [95% CI, 0.31-0.69; P < .001], which is significant. [Although the data were immature at the cutoff, the HR for OS (overall survival) was 0.89 (95% CI, 0.48-1.65).]3,4 PFS analysis based on RET mutation status, specifically the M918T mutation, favored vandetanib as well.3
However, adverse events [AEs] were common, with the dose interruption rate at 47%. The dose reduction rate was 36%, and [approximately] 12% of patients had to discontinue vandetanib altogether due to AEs. The most common AEs were those affecting the GI [gastrointestinal] tract [such as diarrhea and nausea] and hypertension.3
What is the evidence for the use of cabozantinib?
A similar trial was completed with cabozantinib in patients with metastatic MTC a couple of years after the ZETA trial. Roughly the same number of patients [330] were enrolled in this study, [phase 3 EXAM trial (NCT00704730)], comparing cabozantinib with placebo. The primary end point was PFS, and it was highly statistically significant [HR, 0.28; 95% CI, 0.19-0.40; P < .0001]; the median PFS was 11.2 vs 4.0 months, with an ORR of 28% [P < .001], which were all partial responses.5 There were also differences in the response based on the RET M918T mutation status [34% for RET M918T positive vs 20% for RET M918T negative].6
AEs were also common with cabozantinib. As you know, cabozantinib can be a tough drug to give. There were many GI AEs such as anorexia, nausea, and others.6 In this trial, 82% of patients had to undergo a dose reduction, and just under half [46%] also underwent a second dose reduction. I believe [approximately] 16% had to discontinue the study drug altogether.
What is the evidence for the use of selpercatinib?
Some updated data from the LIBRETTO-001 study [NCT03157128] were presented at [the American Society of Clinical Oncology Annual Meeting] this year.7 This trial enrolled [746] patients with non–small cell lung cancer, as well as RET-mutant MTC and a few patients with RET fusion–positive thyroid cancers.8
The study had 3 patient cohorts with RET-mutant MTC: [a treatment-naive cohort, a cohort of patients with previous cabozantinib and vandetanib treatment, and a cohort of patients with previous other treatments]. There were also 2 cohorts of patients with RET fusion– positive thyroid cancer: treatment-naive [patients] and those previously treated. Essentially all patients were metastatic at entry.7,8
The ORR was quite high, 69% [95% CI, 55%-81%] in the 143 patients who’d been previously treated, and 71% [95% CI, 62%-80%] in the treatment-naive group; that is, as a first-line treatment. For the RET fusion–positive [patients with] thyroid cancer, the ORRs were very similar, [77% vs 92% in previously treated and treatment-naive groups, respectively]. Although the median follow-up time is still a little short, most of the groups have very high 1-year PFS rates and ongoing response rates [94% for RET-mutant MTC and 91% for RET fusion–positive thyroid cancer].7
In terms of AEs, [approximately] 28% of patients had at least a grade 3 AE on the study. A lot of those [AEs] were hypertension, some liver functional test [LFT] abnormalities, and a small number [of cases of] diarrhea.7,8 There are some AEs with the drug, but it was not terribly common to have a severe grade 3 or 4 AE.
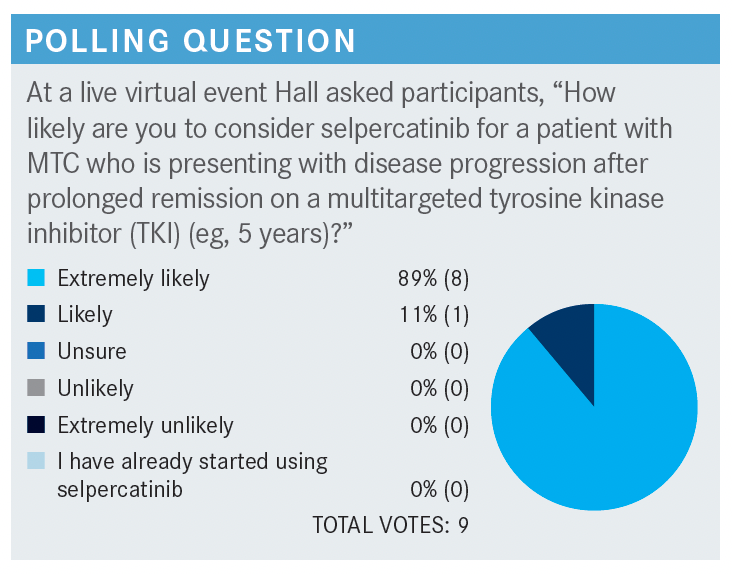
How likely are you to consider selpercatinib for a patient with MTC who is presenting with disease progression after a prolonged remission on a multitargeted TKI?
Let’s say that they have the more common RET M918T mutation. Most [clinicians] would be extremely likely, or likely, to recommend selpercatinib, and I think that this drug, from a toxicity and efficacy standpoint, is highly effective.
What is the evidence for the use of pralsetinib in this patient population?
The phase 1/2 ARROW study [NCT03037385] assessed the efficacy of pralsetinib in patients with RET-mutant MTC. The patient cohorts were very similar to those in the selpercatinib study described above, with patients who had been treated with cabozantinib or vandetanib, an untreated group, those who were treated with something other than cabozantinib and vandetanib, and a group with patients with other RET-altered tumors, typically fusions.9 The patient characteristics were similar to the other studies described above, with patients typically in their late 50s, early 60s.10
The ORR after prior cabozantinib or vandetanib was 60% [95% CI, 46%-73%]; it was slightly higher for the treatment-naïve patients [66%; 95% CI, 46%-82%]. There were quite a few patients who achieved a partial response [58% in the previously treated and 55% in the treatment-naive groups], and I think 2 or 3 had a complete response in the untreated group. The median duration of response had not been reached, and the duration of response was greater than 6 months.10 As for AEs, the hypertension rate was a bit high [40%]. Other AEs were stomatitis, neuropathy, and musculo-skeletal pain, which we didn’t see with selpercatinib.
Pralsetinib was approved in December 2020 for RET-altered thyroid cancers. There were a couple of cases of patients with MTC who were treated with pralsetinib and had tumor lysis syndrome [TLS]. So, there is an addition in the label advising careful monitoring of patients with very high tumor burden.
Does the tumor lysis syndrome (TLS) addition to the label affect your decision-making for these patients?
I would tend to agree with that. I think it might be easy to just monitor their blood work. For patients, that means they have to come to clinic more often; there might be more frequent blood draws for monitoring. With it being in the label, I think you have to watch after these patients fairly closely, but, honestly, I think I would probably do that if they had a high disease burden on selpercatinib as well. Just because we saw it in pralsetinib doesn’t necessarily mean it wouldn’t also happen [to] other patients if you used selpercatinib.
I don’t know, either, what to make of it. It seems [as though] it just might be the patients with high tumor burden who saw rapid reduction in their tumor size and experienced a lot of cell death very quickly. But that could happen on selpercatinib as well.
How likely are you to consider pralsetinib for a patient with MTC who is presenting with disease progression after prolonged remission after a multitargeted TKI (eg, 5 years or longer)?
Most [patients] are extremely likely to use pralsetinib; [I have] maybe a little less enthusiasm for pralsetinib vs selpercatinib.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More