Roundtable Discussion: Persky and Participants Review CAR T-Cell Therapy in DLBCL
Eleven months after completion of therapy with the R-CHOP regimen, a 43-year-old patient with diffuse large B-cell lymphoma presented with fever, drenching night sweats, and recurrent back pain.
Daniel Persky, MD


During a Targeted OncologyTM Case-Based Roundtable event, Daniel Persky, MD, associate director of Clinical Investigations, The University of Arizona Cancer Center in Tucson, AZ, discussed the use of FDA approved chimeric antigen receptor (CAR) T cell therapies to treat a 43-year-old patient with diffuse large B-cell lymphoma.

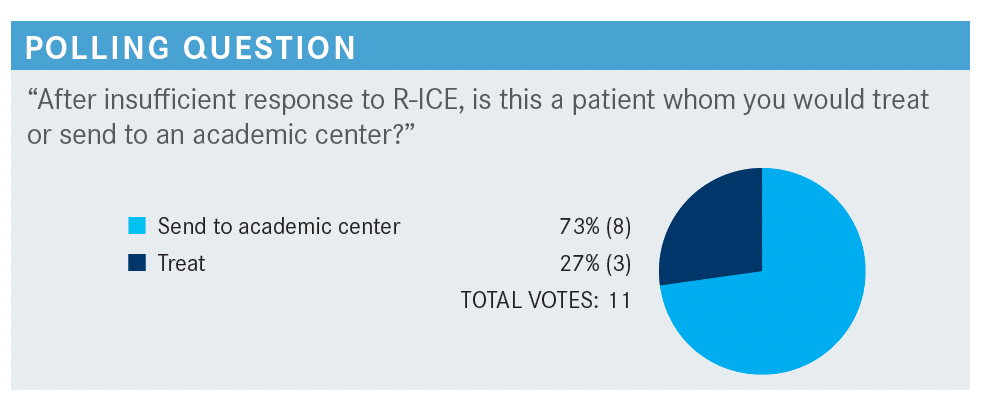
PERSKY: What kind of patients do you usually treat in your practice vs which you usually send to academic centers?
SUD: I would send [a patient such as this] to an academic center, considering even after appropriate therapy with R-ICE, the patient had a durable response. At this point, the [treatment] options are limited. In a community setting, we could probably use GDP [gemcitabine, dexamethasone, cisplatin or carboplatin] or GemDOx [gemcitabine, oxaliplatin, dexamethasone]. [Aside from these], there are not any other great options. The patient would need treatments that are only available in clinical trials or other inventions that would not be possible in a community setting.
GALAMAGA: I disagree. We have new drugs [targeting] CD19 and CD79 pathways that are options if the academic center did not have a clinical trial available and sent the patient back [to the community setting]. In the absence of a clinical trial option, for a patient like this, who has refractory disease, we could consider polatuzumab vedotin [Polivy] or tafasitamab [Monjuvi] as potential options; tafasitamab with lenalidomide [Revlimid] or polatuzumab with bendamustine. Unless we were still trying to push for transplant with an aggressive regimen.
GOLDFARB: I agree that treating with a CD79- or CD19- [targeting agent] is an option.
SUD: As this patient is 43 years old, I would say [treating] aggressively to get the patient into remission, then to a transplant, would be a favorable approach. Although these [targeted agents] are available, I do not have experience with them, so I would prefer sending this patient to an academic center.

PERSKY: We discussed some [guideline-recommended] therapeutic options, including polatuzumab-BR [polatuzumab, bendamustine, rituximab] and tafasitamab plus lenalidomide.1 Would anyone consider CAR [chimeric antigen receptor] T-cell therapy at this point?
PARK: [Access to CAR T-cell therapy] would be a large motivator to send the patient to an academic center, or a center that does CAR T-cell therapy.
PERSKY: Do you think the goal of therapy for this patient is curative, or do you think it is palliative?
YOO: If the patient cannot safely get an autologous stem cell transplant and is not able to reach a second complete response [CR], then I usually start mentioning that the therapy could be palliative, unless there is some great treatment option available.
PARK: It depends on what effect CAR T has, because even if the patient [does] not achieve a CR, they may have some level of durable remission. It is hard to say that treatment is just palliative at this point. [Someone with refractory DLBCL is] not exactly the equivalent of someone who has, [for example], a stage IV solid tumor cancer.

MUKHERJEE: It depends on the tumor burden. This patient had high Ki-67 [expression], so pretty aggressive disease. If [manufacturing] the CAR T-cell therapy was going to take a while, it would be plausible to have a bridging therapy. Whether I would use a CD19 antibody for bridging therapy would depend upon [if I thought this therapy would be needed at another time]. I would prefer to reserve anti-CD19 therapy for use when it is most efficacious.
PERSKY: That’s fair. You have to think about the choices of bridging therapy if you are considering [using it].
CHERINGTON: I have not had too many patients [in whom] I have used bridging therapy, so I do not have a lot of experience with that, but I think there is a place for it. In some patients, I may try to debulk [the tumor] slowly over time, or just to maintain it, to get the patient to a point where they would be a better candidate for CAR T or stem cell transplant.
PERSKY: Yes, some of our patients are progressing so quickly that we are concerned that they do not have enough time [to wait] for the manufacturing of the CAR T cells. So we need to keep a lid on the disease, not necessarily to cause a great response, but at least to prevent further progression and deterioration [in the patient’s condition], because that is sometimes the reason why patients cannot proceed with CAR T-cell therapy.
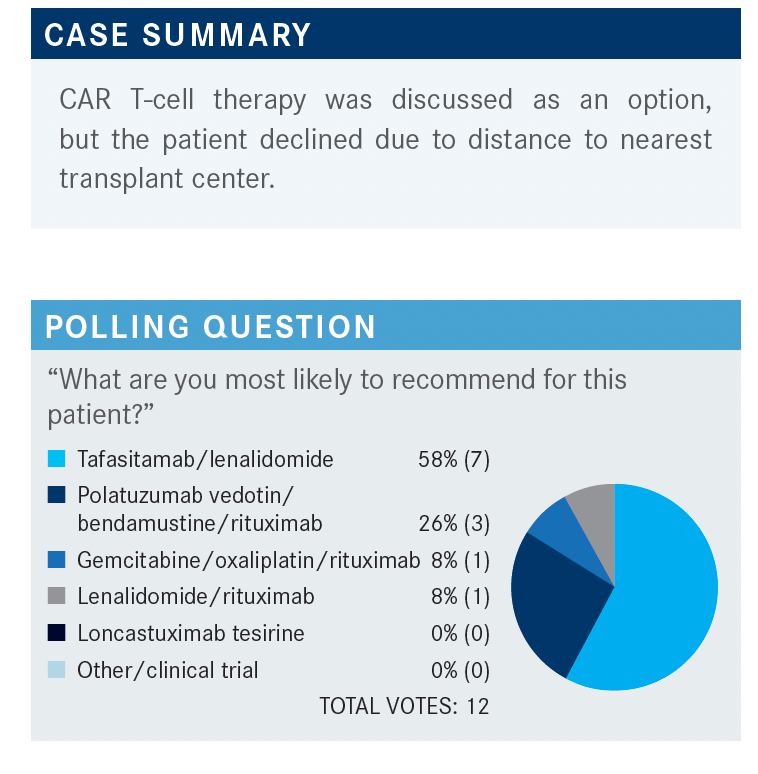
PERSKY: Let’s discuss our choices in this case. What factors influenced your decision to make one therapy recommendation or another?
PARK: I picked tafasitamab and lenalidomide because I recall a talk I attended [where they reviewed trial findings showing] CR rates and durability of response that were impressive, considering the patient population. At the same time, [I would also] consider a clinical trial, but I would want to get some level of response in this patient, so considering multiple options would be the way to go.
KHATTAB: I also chose tafasitamab and lenalidomide. I have experience using this combination [to treat] an 86-year-old woman, and she did amazingly well on it. [Other factors to consider include] insurance issues and the patient’s comorbidities, but I find this combination is effective and well tolerated.
KAHN: I have given both of the top 2 options, although my use of these therapies was more dictated by insurance and cost than anything else. [Nevertheless], I had good responses with both of them. One patient did not get the bendamustine[-based therapy] because their ultimate goal was to get CAR T-cell therapy.
GOLDFARB: I do not think that chemotherapy is going to be very effective [in this patient] if they failed R-ICE, so I think you have to move on to new drugs, not to more chemotherapy.
GALAMAGA: My preference would be to avoid adding bendamustine, as the patient has already had treatment and could develop long-term toxicities. The patient is only in her early 40s, so I would want to get her to the point where she had clinical trial options, maybe even after she has had a good response [to therapy]. I chose the tafasitamab option for her as well. I have had experience [with this drug], and the tolerability is excellent.
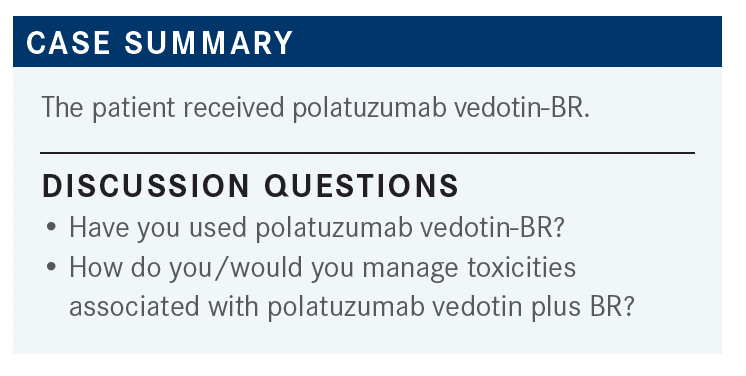
PERSKY: If you have used it, what was your experience?
GALAMAGA: I have experience using polatuzumab-BR [and have found that patients have] a decent response, but some patients experience toxicities, mostly to the bendamustine.
ARSLAN: The progression-free survival is only [several] months with polatuzumab plus BR,2 so it is less efficacious [than tafasitamab]. [For therapeutic] bridging to CAR T-cell therapy, patients could be given polatuzumab plus rituximab, and once the CAR T cells are collected, you can add bendamustine to the treatment. I have not done it [myself].
PERSKY: I [have] wondered [about] this myself, but I have not done it.
If you have had experience with polatuzumab-BR, how have you managed the toxicities? If you have not, what would you do?
VICUNA: I have not used polatuzumab-BR, but I would [reduce the] dose to manage cytopenias and maybe hold [the patient off the treatment] cycle an extra week—so every 21 days, give them an extra week off to recover. To manage peripheral neuropathy, I may hold [the patient off treatment] and lower the dose once the toxicity has improved.
ARSLAN: If the patient has been exposed to many chemotherapies, you can initially give bendamustine at a lower dose. We do not have to [start bendamustine at a dose of] 90 mg or 100 mg, we can start at a dose of 70 mg, then give the patient G-CSF [granulocyte colony-stimulating factor], if needed.
PERSKY: Would you say you might do this routinely or for just older patients, where there have been concerns about the 90-mg dose?
ARSLAN: A patient who gets 2 or more lines of chemotherapy is going to be neutropenic. I would start the patient at a lower dose and give them G-CSF. The National Comprehensive Cancer Network guidelines recommend that, even if the patient is [at] intermediate risk for neutropenia, you can use G-CSF.1
PERSKY: Right, especially with COVID-19.

GALAMAGA: In terms of pros and cons, I honestly do not know enough about the biology [to comment on which therapy is] superior in each context. Some of us mentioned tafasitamab, and I have been very encouraged by my experience with this drug and the emerging data on it. Fixed-duration therapy appeals to [younger] patients who want to see the end point of finishing therapy and moving onto observation rather than accepting [treatment as] a lifelong process that has to be maintained and controlled.
In terms of [the role of] polatuzumab-BR in the CAR T-cell landscape, there is such an art to oncology in this context. There are so many compounds [for DLBCL], so sequencing therapy is an important discussion to have. [It’s important to involve] our clinical trial experts in these discussions and to try to prioritize getting patients to those CAR T-cell options. Maybe 1 of these novel therapies [could be a bridging option for CAR T-cell therapy], but we need some more guidance on that.
PERSKY: I agree. We have had 3 new CAR T-cell products and 4 drug approvals for relapsed DLBCL in the past 4 years.
PERSKY: Do you see differences [regarding the] receptor the agent is targeting or in the value of fixed-duration vs outpatient treatment? Do you think of [these factors] differently, depending on whether the patient is a candidate for transplant?
NATH: If you are thinking of CAR T-cell therapy [for the patient] in the future, the question is whether you want to give them another CD19-directed therapy; [the data are not clear on this]. The responses seem to be higher using this approach, [so I keep this in] the back of my mind as I make [treatment decisions]. If the patient is not going to proceed to the next step of treatment, such as transplant, you have to stop it at some point, especially if [the patient has had] a response.
I am not sure I would make treatment decisions based on [whether the patient is a candidate for] transplant or not. Either way, either of the combination therapies can be used. Getting the CR is important, especially if you are looking to take the patient to transplant in the future. The shorter time to CR, the better.
YOO: There is some question [about whether] we should use anti-CD79 therapy for bridging [or whether] we can safely use tafasitamab, which targets CD19. I generally [avoid using] any anti-CD19 agent in patients who are candidates for CAR T. Otherwise, both [targeted therapies] are great options. I do not think anyone knows which one should be [used for] third- vs fourth-line [treatment] if we are not thinking about CAR T, but until we know more about it, [I don’t think we] should use any CD19 blocking agent for CAR T candidates.
PERSKY: Does it matter whether the patient is a transplant candidate?
YOO: If the patient is not a transplant candidate, [I would take] more of a palliative treatment approach. Statistically, [these patients are more likely to be] frail and heavily treated, so I would pick something easier [for them to take]. Findings from the L-MIND trial [NCT02399085] showed tafasitamab had good tolerability.3 But again, I do not have much experience with either [tafasitamab or polatuzumab].
KAHN: I have had good responses with both [therapies], and the decision to use one vs the other seems to [depend] more on cost and insurance issues than anything else. But none of the patients I‘ve used these therapies in were transplant candidates, for various reasons. [Rather], they were candidates for CAR T-cell therapy. I have not used [these therapies] in the setting of transplant candidates.
PERSKY: What do you think the remaining unmet needs are for patients who don’t want, or can’t receive, CAR T cells, or have disease that relapsed after CAR T-cell [therapy], or even after transplant? Have we met the needs, or are there unmet needs remaining?
KHATTAB: We still have a ways to go with DLBCL, even with CAR T cells. Access to CAR T cells is still a major challenge. I work in Phoenix, Arizona, and I can hardly get anybody into an evaluation for CAR T cells. I have been successful only once so far. The patient [who received it] got it on a clinical trial and, unfortunately, died from COVID-19. So access [to CAR T-cell therapy] is still a major issue.
I really like tafasitamab. That combination is effective and well tolerated. Technically, tafasitamab is not chemotherapy, and it seems to me that [targeting] CD19—this is just a general impression—is more effective than [targeting] CD79. Going after CD19 makes sense.
YOO: For a patient with non-GCB [disease], ibrutinib [Imbruvica] seems to be an option, but where does it [fit in with] all these new therapies?
PERSKY: That is a great question. I personally have not used much single-agent ibrutinib for non-GCB [disease] because it is [associated with only] a short duration of remission. I have used it as part of combination therapy with lenalidomide and rituximab, a regimen called IR squared.
But considering the new, improved combination therapies, the place of IR2 is [waning], particularly because [it is associated with] high-grade rashes; 16% [of patients treated with IR squared had a] grade 3 rash.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. B-Cell lymphomas, version 5.2021. Accessed October 5, 2021. https://bit.ly/2YoPUR8
2. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
3. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More