Roundtable Discussion: Rizzieri Compares Benefits and Challenges of Tafasitamab in Patients With DLBCL
One year after a 75-year-old patient with diffuse large B-cell lymphoma achieved remission following 6 cycles of R-CHOP, the patient relapsed but declined transplant.
David A. Rizzieri, MD

David A. Rizzieri, MD, professor of Medicine, chief, Section of Hematologic Malignancies, clinical vice-chief, and associate director for Clinical Research at Duke Cancer Institute, Duke University School of Medicine led a group of peers in a discussion about a 75-year-old man with diffuse large B-cell lymphoma.
RIZZIERI: [Approximately] half of our patients after treatment with R-CHOP relapse, and so this issue of rechallenging with rituximab is common. Are you comfortable rechallenging with a rituximab-based regimen if the patient progresses, let’s say, within 6 months vs 3 years later?
WEIL: In the primary refractory setting, where I’m giving them active rituximab-based therapy and we have refractoriness, I do not. Because I don’t feel there’s great utility in that. If you are relapsed...6 months later, something like that, then, yes, I do recycle rituximab with my salvage regimen.
RIZZIERI: Is that a similar practice pattern? I think it’s very reasonable.
RUDOLPH: Most patients will need another chemotherapy to bridge them to transplant or CAR [chimeric antigen receptor] T-cell therapy or to just a follow-up if patients are not a candidate for either.
RIZZIERI: I think they need a different regimen, and if targeting CD20 with rituximab is failing them, an alternative is certainly appropriate. I think Dr Weil mentioned [that] this issue of progressing while on therapy as opposed to progressing years later certainly can color our thoughts about rechallenge as well.
In terms of what that alternative regimen might be, what [influences] your thoughts? There are a few things [to consider for alternate therapies]: the chance of another regimen giving a durable response, safety/tolerability, comorbidities, and patient preference. Is there anything in particular that makes you choose one regimen over another?
TOLBERT: We used to use RICE [rituximab, ifosfamide, carboplatin, etoposide]...but it’s a more difficult regimen to give, and I like giving R-GDP [rituximab, gemcitabine, dexamethasone, cisplatin]. I would be hesitant to give platinum to this patient, secondary to their renal dysfunction.
PATI: All of these factors [that you mentioned] are important: chance for response, safety and tolerability, comorbidities, and patient preference. I’ll use the regimens if the patient can tolerate them; depending on the patient choices we might even offer [a less aggressive regimen].
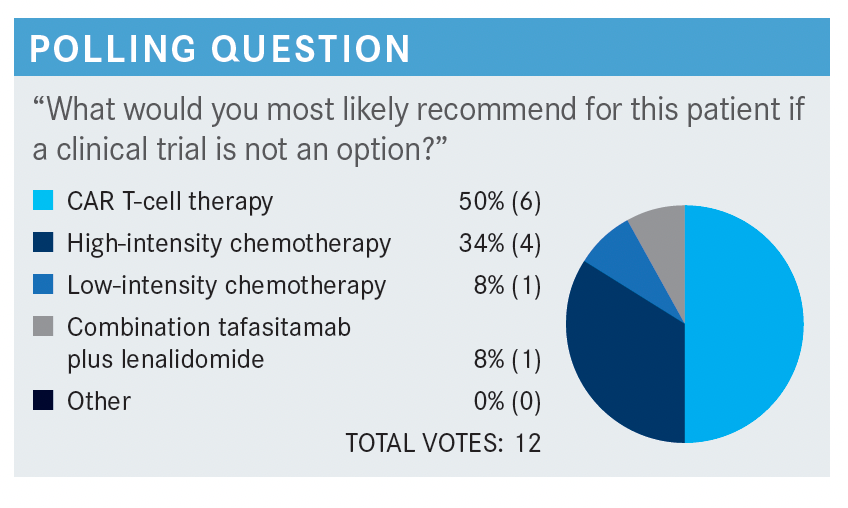
RIZZIERI: I’d be interested in the group’s [opinion on] the efficacy of tafasitamab [Monjuvi] and lenalidomide [Revlimid]. Any thoughts on that in particular?
PARIKH: I think [it is a] very effective regimen. I think the CR [complete response] rates are very impressive, especially in the patients who are primary refractory.1,2 I think it’s really an attractive option, at least for that group of patients.
RIZZIERI: I think the [patients who are primary refractory] are in such need, and I’m encouraged to see that as well, so I agree. Other thoughts on the efficacy data?
WEIL: I’m impressed by duration of response, and I think that that’s meaningful because in a palliative setting with chemotherapy for DLBCL, we know not all DLBCLs are created equal.1,2 On average, your duration of response is short-lived; a year, maybe a year and a half if you’re lucky, and you end up with [patients] relapsing. So to see a duration of response, for those who respond, going out to multiple years is remarkable. And that is beneficial to this subset of patients who may not have high-dose or curative options available to them.
RIZZIERI: What’s most striking [to you about the safety data for tafasitamab and lenalidomide]?
MORAVINENI: Certainly, [the] safety data are troubling, as you have several grade 3 adverse events [AEs]. Of 80 patients in a clinical trial, 12% discontinued [due to treatment-related AEs (TRAEs)]. That means in a community setting it’s probably 20% plus. That is troubling to me. I agree, [in patients who are primary refractory], I don’t have a doubt [about using it], but remaining groups I would be very cautious [about using] this.
RIZZIERI: Which [TRAEs] in particular, in the community setting, are more troubling to you?
MORAVINENI: I know neutropenia we can monitor, I’m not too bothered, although I suspect sepsis-related deaths or hospitalization is a major issue for these patients. But it’s a whole list of things, not one. [There are] several toxicities to monitor, and [it is] not an easy regimen, [with a] significantly high [discontinuation] rate and very high cost for a noncurable disease. If [a patient has] good [ECOG] performance status, is 50 to 60 years old, I don’t have much problem. But if [patients have] more comorbidities, which is common in the community setting, [the safety data are] really troubling.
RIZZIERI: What regimen in your practice are you offering?
MORAVINENI: I may go for a clinical trial, I may go for low intensity, I may go for bortezomib [Velcade], [or lenalidomide]-based regimens. They have very acceptable toxicity and reasonable benefit.
RIZZIERI: What [do] the others think, in terms of acceptability in your practice population, [do you have] difficulty getting patients through?
KOFF: So, for me, I’m not too alarmed by this toxicity profile. I think most regimens, even the palliative regimens that you use in this setting, are going to have similar profiles of toxicity. Before tafasitamab plus lenalidomide, in my older [patients with] DLBCL who were not candidates for transplant or CAR T, I’ve been using R2 [lenalidomide plus rituximab] quite a bit, and I think this is similar but with the added punch of much better efficacy. The safety data, for me, are not what keep me from prescribing this more often.
I think it’s more finding the right patient who you are not potentially getting ready for a CAR T, and so you’re worried about CD19 expression. And, in the noncurative patient, [it means] balancing quality of life not with AEs, per se, but with infusion center visits every 2 weeks indefinitely.
RIZZIERI: I think that’s a research effort that is under way right now, and to your point that none of these treatments are cheap, it is just as important.
It’s interesting you bring up the toxicity, what alternatives we might use, and [whether we should be] thinking about it. Because, as Dr Koff brought up, R2 is something that is not uncommon in this setting. So what would happen in this population [taking] tafasitamab plus lenalidomide if they were just to get lenalidomide alone, which is sometimes tried? We don’t have the head-to-head study, but we do have the RE-MIND study [NCT04150328], which is a retrospective study with a propensity-score–based 1:1 matched comparison.3
I really like the propensity score. I challenge my inner groups that I work with to really do the work and do [a] propensity score rather than just these meshed historical controls. Because the propensity score tries to account for the different comorbidities in the populations and our proclivity to suggest one regimen over another because of differences in the population. And so, this is at least an attempt to account for differences between patients who may get tafasitamab plus lenalidomide vs lenalidomide alone.
Covariates were assessed in terms of prior therapies, patients getting the combination vs lenalidomide alone, and...overall response rates between these groups. There’s a suggestion [in the results] of the [patients taking] tafasitamab plus lenalidomide of having a higher CR rate and a higher overall response rate.3 That shouldn’t necessarily surprise us—it’s an effective drug. I think the key would be [asking whether it comes] at too much cost; meaning, real cost as well as safety.
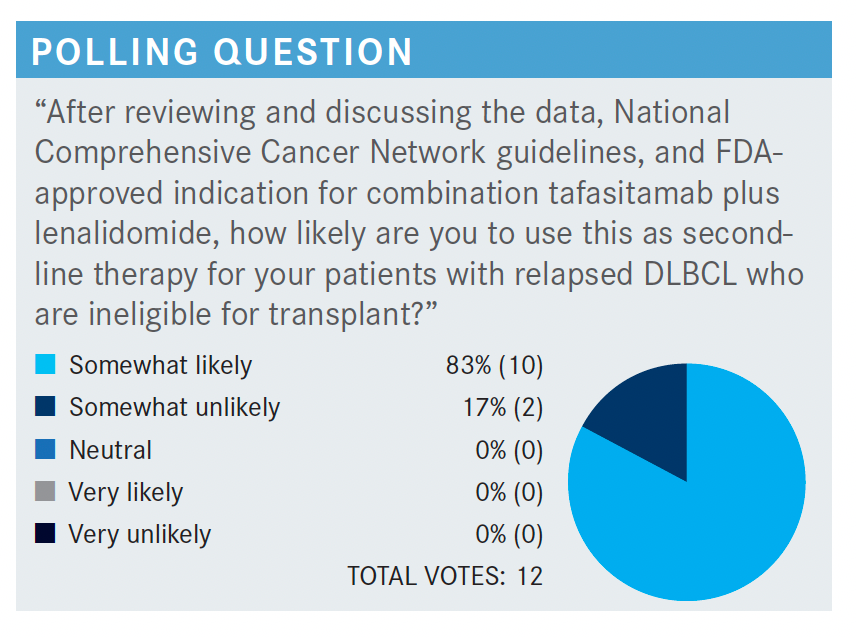
PARIKH: In regard to the neutropenia, I’m assuming we don’t use growth factors? In that case, would you use the prophylactic antibiotics because it seems [as though] the rate of neutropenia is pretty high?
RIZZIERI: You would follow your personal practice guidelines; there’s nothing absolute there, but, personally, I do. I’m a believer in antibiotics to try to prevent emergency [department] visits and the misery that comes along with that, so that’s my practice pattern. And then, frankly, I’m very comfortable with dose reduction of the lenalidomide. I think it starts at a high dose, and we can very readily dose-reduce to try to minimize that neutropenia.
PARIKH: Do you think that most of the neutropenia is from lenalidomide, or just the combination, or the disease, or multiple factors?
RIZZIERI: Most AEs were well managed with lenalidomide dose reduction. So [although] the combination is in part to blame, the vast majority of patients are well [treated] with reducing the lenalidomide alone.
TOLBERT: Why would anyone start patients on a high dose of lenalidomide in a regimen where you know it’s going to have a high predisposition to febrile neutropenia and, certainly, neutropenia? I would not start anyone on this regimen with a high dose of lenalidomide. I don’t understand the point of that. Are we really going for that much dose intensity, or does that dose intensity make a difference for these patients? We’re not doing that with [patients with] myeloma.
RIZZIERI: I’d be curious what others think about that comment, any other thoughts on that? And to your point, is the dose intensity of lenalidomide really justified in this patient population?
PATI: Because it’s a combination of lenalidomide with a monoclonal antibody, it’s not unreasonable. Think about a regimen [such as] RICE, which is intensive in terms of neutropenia and we are OK with that. So, I can only say [that it is reasonable for me] to start with, and that you dose reduce when you need to.
OSEI-BOATENG: I agree that knowing up front that you have a fair amount of neutropenia and other cytopenias, I don’t think it’s unreasonable to start with a dose reduction, something [such as] 20 [mg]. Because you’re more likely to do it in [that] combination. And in my practice, I dose-reduce a fair amount with lenalidomide. I haven’t used this, but looking at the data, it seems [as though] the type of patients I would use this on, I can predict that they wouldn’t do very well at 25 mg, and [so I would] start at 20 mg. I don’t think I’ll lose that much efficacy going that route.
RIZZIERI: And, if I recall the data from L-MIND [NCT02399085], the majority did have a dose reduction, but [approximately] 70% were able to tolerate 20 mg for 3 weeks of the cycle.1,2 But you’re preaching to the choir. I’ve often felt that the whole point of targeted therapy is to get something on board at a low dose that they can stay on for a long time, rather than [on then off again] from the toxicity. I don’t disagree with the concern that was raised about the dosing there, either.
So, on-label was the dose chosen, but I don’t disagree with the concern and with the toxicities that we have to manage, I think the real key is following the patients closely with their counts and dose reducing [as] appropriate, if you do choose to start at that higher dose. And that really speaks to the next point of barriers to using it, in terms of the dosing and the AE profile.

SCHWARTZ: I haven’t been in this situation too often. I have been comfortable sending these [patients] for CAR T-cell therapy in that setting because I think there [are] still, mechanistically, other things going on that may give these patients a more durable response. I think trying to beat them up with more chemotherapy is very challenging.
RIZZIERI: I know the agent hasn’t been out all that long. I’ve had the converse, but I haven’t had the situation of tafasitamab, then CAR T, myself.
REFERENCES:
1. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. Accessed October 12, 2021. doi:10.1016/S1470-2045(20)30225-4
2. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. Accessed October 12, 2021. doi:10.3324/haematol.2020.275958
3. Zinzani PL, Rodgers T, Marino D, et al. RE-MIND: Comparing Tafasitamab + Lenalidomide (L-MIND) with a Real-world Lenalidomide Monotherapy Cohort in Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin Cancer Res. October 7, 2021. Accessed October 12, 2021. doi:10.1158/1078-0432.CCR-21-1471
