Roundtable Discussion: The Role of Adjuvant Therapies for NSCLC Based on Disease Stage
During a Targeted Oncology case-based roundtable event, Joel Neal, MD, PhD, and Leah Backhus, MD, MPH, discussed adjuvant therapies including chemotherapy, immunotherapy, and targeted therapy for patients with non–small cell lung cancer.

Joel Neal, MD, PhD (Comoderator)
Associate Professor of Medicine (Oncology)
Stanford Medicine
Palo Alto, CA

Leah Backhus, MD, MPH (Comoderator)
Associate Professor of Cardiothoracic Surgery (Thoracic Surgery)
Stanford Medicine
Palo Alto, CA


NEAL: As medical oncologists at the Stanford Health practice, our clinics are fairly busy, and patients are only referred [by surgeons] when there is a [specific] reason. Dr Backhus, what are the considerations you think about [as a surgeon]? How do you set up that discussion?
BACKHUS: I think it’s helpful for patients to have discussions with their physicians and feel like they’re getting the full breadth of treatment options. Whether or not they ask for it, I think that they all find it helpful. I do try not to [overwhelm] my colleagues’ oncology panels with patients with stage IA cancer. I definitely refer patients with stage II cancer, and I also refer patients with stage IB cancer that is accompanied by high-risk features.
NEAL: It’s no accident that in the new staging criteria, stage IIA is [tumor size] over 4 cm.1 We know that patients with stage II cancer and higher should get adjuvant therapy. [Regarding the question of] whether or not to refer, [I think] that patients with stage IB or stage II cancer should definitely be referred.
We’re all taught “cisplatin, all the time, for everybody.” Dr Arslan, when you’re thinking about [adjuvant] chemotherapy in non–small cell lung cancer [NSCLC], how often do you actually give cisplatin vs carboplatin-based therapy in the adjuvant [setting]?
ARSLAN: Most of the time, if the patient is fit enough to tolerate such a big surgery, they are fit enough to tolerate cisplatin. So I try to give cisplatin to all the patients whose cancer is stage II or higher. With stage IB, I also take into consideration [the high-risk] features. That being said, I think there may be less of a role for adjuvant chemotherapy now because we are giving more and more neoadjuvant chemotherapy. [For example], when giving 3 cycles of neoadjuvant chemotherapy with immunotherapy, we don’t have to worry about giving 4 to 6 cycles in the adjuvant setting. And also, if the patient is negative for EGFR mutations, I don’t have to worry about giving 1 year of a PD-1 inhibitor. I think in the future the role of adjuvant chemotherapy will change drastically.
NEAL: Interesting. It sounds like you’re in favor of more neoadjuvant therapy, as long as you’ve seen the patient beforehand. You might, in parallel, refer to a surgeon and also set up the patient for chemotherapy. Is that a fair assessment?
ARSLAN: Yes, I think if you can give just 3 cycles instead of 1 year of treatment, that is a better approach.
NEAL: Yes. The CheckMate 816 study [NCT02998528] did have variable adjuvant therapy. Does anybody else have an opinion on this?
LASHKARI: I think that the use of neoadjuvant chemoimmunotherapy is going to change the dialogue, because we know that adjuvant platinum-based chemotherapy does not significantly reduce the absolute risk of recurrence.2
Greater response and better outcomes may be achieved by providing neoadjuvant treatment [and leveraging a] different mechanism of action, leading to patients achieving a state of no evidence of disease, rather than by going to surgery first and then trying to achieve additional benefit afterward. I think this is where the discussion needs to go.
NEAL: Yes. I think now that we have a neoadjuvant approach and an adjuvant approach, it’s going to get trickier to decide which one to pick for individual patients. Multidisciplinary discussions with great teams are always the right answer to these things.
FAHED: Is neoadjuvant therapy now reserved for stage III?
NEAL: I’m tempted more to use it in stage III. Most of our patients, though, who are potentially surgically resectable see a surgeon and get resected. There are pros and cons to neoadjuvant and adjuvant therapy. With adjuvant therapy, all the patients go to surgery first and then they get treatment later.
With neoadjuvant therapy, all the patients get chemoimmunotherapy first and may not get therapy afterward. If both are beneficial, we have to look at the relative pros and cons. I think it will be a case-by-case discussion. I think we have tended to say that patients with stage III cancer are the patients most appropriate for neoadjuvant therapy, even if they may be resectable later.
CHAND: What are you doing for those patients who have an ALK or a KRAS mutation and have stage IIIA [disease]?
NEAL: [It is] always important to know at what point we figured out about the ALK and the KRAS mutations. The KRAS mutations are often associated with smoking, and they can respond to immunotherapy. I just follow the immunotherapy algorithms. I don’t give sotorasib [Lumakras] and other things as first-line treatment in stage IV, so why would I give them as adjuvant therapy?
ALK [alterations are] totally different. When I know that a patient has an ALK or a ROS1 [mutation], I consider those very special [situations]. Even though I don’t prescribe off-label alectinib [Alecensa], a colleague recently emailed me that they have a patient [who had stage III cancer] and who has started adjuvant therapy with alectinib and is going to continue it for 3 years.
We do have a trial of adjuvant ALK-targeted therapy [ALCHEMIST; NCT02194738] that is recruiting, although I will admit that the trial arms are observation vs crizotinib [Xalkori], and crizotinib is no longer a preferred drug [for treating ALK-altered cancer]. I wouldn’t prescribe adjuvant alectinib or other ALK-targeting drugs, even for stage III patients. I would definitely give these patients chemotherapy. That’s where we have the data. In general, I would withhold immunotherapy for ALK-altered cancer but give [immunotherapy] for KRAS-mutated cancer.
CHAND: I have a [patient with a] KRAS mutation who is on adjuvant chemotherapy, and I somehow got next-generation sequencing for him, even though [his cancer] was early stage. But I wasn’t sure if I should give him maintenance immunotherapy or not.
NEAL: With the exception of KRAS G12D, which is sometimes a little less responsive to immunotherapy and is not caused by smoking, most of the KRAS mutations respond pretty well to immunotherapy. I would definitely give atezolizumab [Tecentriq] to [patients with these other KRAS mutations].
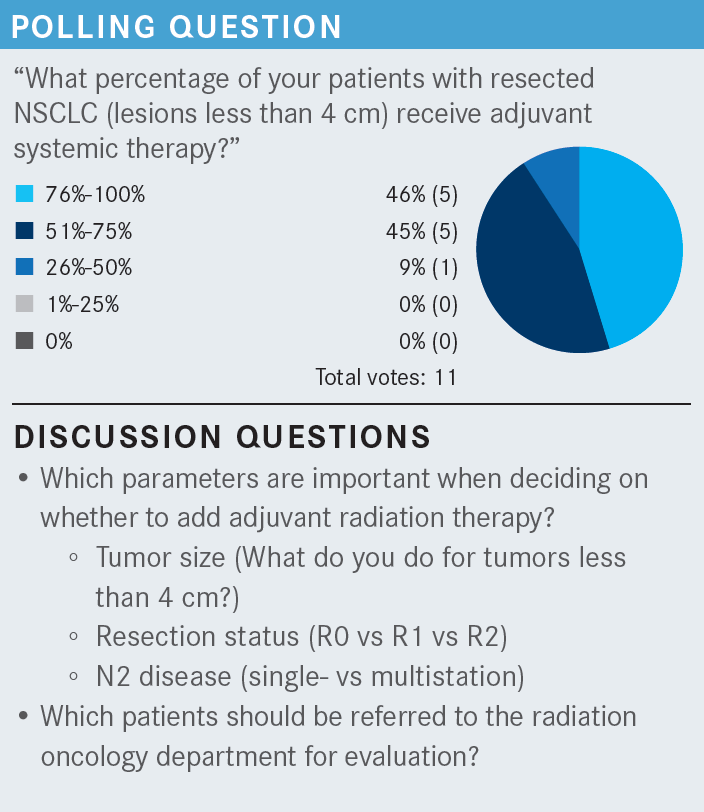
CHAND: I generally keep adjuvant radiation for later, for example, for tumors that have hilar lymphadenopathy. I generally don’t send patients for adjuvant radiation if they have stage II disease. I limit their treatment to adjuvant chemotherapy. If patients are unresectable or have stage IIIB or IIIC disease, then those patients will automatically get chemoradiation.
NEAL: In the case [we are discussing here, the mass measures] 5.5 cm, [and the patient] had surgery [in order] to get chemotherapy. I think most of us would agree that in this case, we don’t need to bother the radiation oncologist.
[Dr Backhus, for which] patients do you think, “I should probably get a referral to radiation oncology [for postsurgical radiation therapy]” based on the pathology report, even before the tumor board discussion?
BACKHUS: I can’t say that that’s in my algorithm up front because I feel that there are still so many unknowns. Not that it’s inappropriate. But I never win that [discussion]. I had a recent discussion about a patient [in this situation, and the radiation oncologists] stood firmly behind their data, which don’t show benefit. But in the case of an incomplete mediastinal nodal dissection, where I know there are positive nodes that I couldn’t get to [and I] know that there’s gross disease, I still think there’s some potential benefit. I think that’s the only time that I’ve had even marginal success in talking with radiation oncology in this setting. With positive margins, that’s an easy sell, but it’s more nuanced [when you have to consider] the lymph nodes.
NEAL: [It’s impressive that] the radiation oncologists [would say], “We can’t do any better. You left the margins negative, and you got out all the nodes. What more can we do?”
BACKHUS: I would put patients [with no positive nodes] in the R1 or R2 resection category, in terms of making the argument to radiation oncology.

NEAL: Adjuvant immunotherapy is more common for us; [neoadjuvant immunotherapy and osimertinib are] a little more unusual because of the patient population. I think we’re going to see more neoadjuvant immunotherapy, but I don’t know that it’s going to displace the adjuvant [immunotherapy].
Here’s one [advantage]: a patient with EGFR-mutant lung cancer who has surgery, [with or without chemotherapy,] who would have qualified but doesn’t get adjuvant osimertinib for some reason. I have had a patient like that. As somebody who has run a large number of these clinical trials over [the course of] a decade, I would say [that approximately] half the patients, when you tell them to take a pill for the next 3 years, say, “I’ve had enough. I went through surgery, I went through chemotherapy, and I just don’t want to do any more.”
When we were running these trials, we tried to keep track of the [total number] of patients that we offered the therapy to, in addition to [the number] who actually went on the study. We don’t [often consider] the number of people who said no to the study. I think it’s great to have a discussion, but with adjuvant tyrosine kinase inhibitors, I feel like patients have stronger feelings. With adjuvant chemotherapy, I’ll tell the patient, “I strongly recommend this for you. You had a stage IIIA lung cancer, but there were 5 positive lymph nodes, and the chance of having positive contralateral nodes or something else is going to be high. We can try to [manage] the disease for the next 3 years, but I can’t guarantee that I’m going to cure it.” That’s how that discussion goes. Whereas with a stage IB tumor that has been completely taken out, that is right on the borderline.
[Patients have to decide whether they] want to be on medication for 3 years or wait and treat the cancer when it recurs. We don’t have the overall survival data showing us where the [Kaplan-Meier] curves come back together. Three years later, they look great, but what about 6 years?
LASHKARI: The one reassuring thing is that osimertinib, [in comparison with] its predecessors, has less toxicity.5 In particular, rash and diarrhea don’t tend to be as significant. I haven’t treated any patients per the ADAURA trial, but I’ve had patients on osimertinib for 1 to several years in the metastatic setting, and they’ve largely tolerated the medication very well. I think that makes it a slightly easier sell.
If the adverse events were significant or chronic, that would make long-term treatment challenging. With all adjuvant trials, there’s always some empirically based time frame given for how long a patient should be on therapy. Unfortunately, this often [is simply derived from] the design of the study, so it is unclear whether 3 years is absolutely necessary.
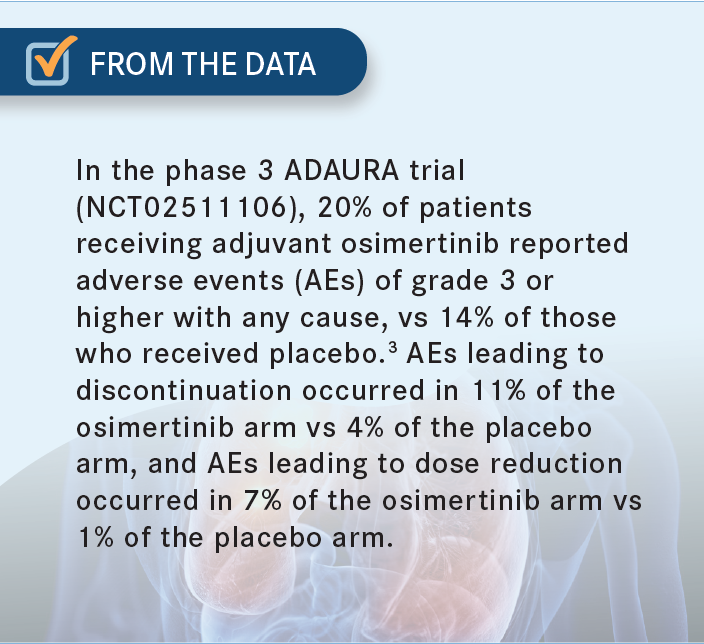
NEAL: I completely agree with both of your points. I ran not only an adjuvant erlotinib [Tarceva] study and treated patients myself, but also an adjuvant afatinib [Gilotrif] study, which was designed to [address] this question of [whether] longer [treatment] is better. It’s one of the only studies that actually answered that. Even though [the study] didn’t make its primary end point of showing a better disease-free survival for 2 years, there was a difference between the 2 groups of about 10% fewer recurrences [when the drug was taken] for 2 years vs 3 months.6 I think these drugs have a very different mechanism of action. Osimertinib is by far the most tolerable of the approved EGFR inhibitors and has better central nervous system penetration, [though it does carry small] risks of heart problems and pneumonitis [From the Data3]. But 3 years may not be enough [for osimertinib]. We might need 5 or 10 years, whereas [for] immunotherapy, 1 dose may be enough.
AMBIKA: Yes, I was about to ask whether 3 years is enough. My other point is that [osimertinib] definitely reduced the [central nervous system] risk.2 That itself is a big bonus, because previously we had patients return 6 to 12 months after chemotherapy with brain metastases. Those are disastrous.
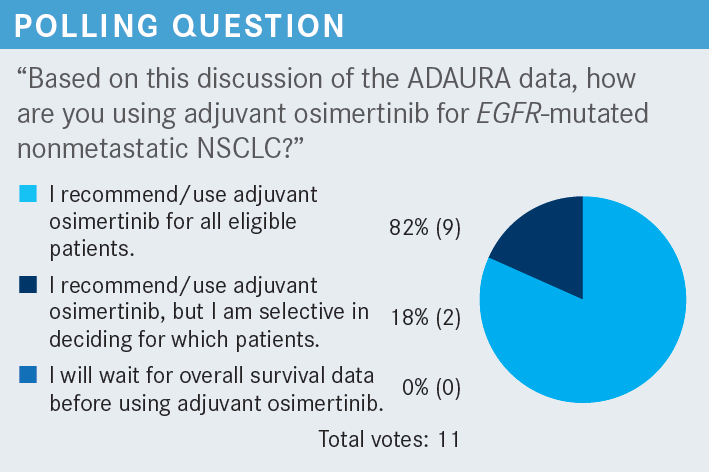
NEAL: I’m selective in deciding which patients should receive it. I feel like I have to be selective. I consider the risks and benefits for each patient. I consider how small the tumor was; technically, eligible patients have tumors that measure 3.1 cm. However, the patients may not want to be on osimertinib for 3 years. It’s a nuanced process of shared decision-making. I’ve had some insurance and reimbursement issues too. I think there has been a transition with respect to the insurance companies. They’ll pay for osimertinib for stage IV cancer, but for stage IB cancer, they [challenge it].
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 5.2022. Accessed October 13, 2022. https://bit.ly/3RPMugk
2. Kreuter M, Vansteenkiste J, Fischer JR, et al; TREAT Investigators. Three-year follow-up of a randomized phase II trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine (the TREAT Study). J Thorac Oncol. 2016;11(1):85-93. doi:10.1016/j.jtho.2015.09.014
3. Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(suppl 18):LBA5. doi:10.1200/JCO.2020.38.18_suppl.LBA5
4. Wu YL, Tsuboi M, He J, et al; ADAURA Investigators. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
5. Tagrisso. Prescribing information. AstraZeneca Pharmaceuticals LP; 2020. Accessed September 28, 2022. https://bit.ly/3yFfvER
6. Neal JW, Costa DB, Muzikansky A, et al. Randomized phase II study of 3 months or 2 years of adjuvant afatinib in patients with surgically resected stage I-III EGFR-mutant non–small-cell lung cancer. JCO Precis Oncol. 2021;5:325-332. doi:10.1200/PO.20.00301
