Dosing and Toxicity: Managing Adverse Events and Dose Reductions for PARP Inhibitors in Ovarian Cancer
During a Targeted Oncology case-based roundtable event, Bradley J. Monk, MD, discussed recommendations for managing toxicity and dosing of PARP inhibitors as primary maintenance for ovarian cancer.
Bradley J. Monk, MD (Moderator)
Professor, Obstetrics & Gynecology
University of Arizona College of Medicine – Phoenix
Phoenix, AZ


CASE SUMMARY
A 49-year-old Black woman presented to her primary care physician with abdominal bloating and nausea. She had a history of mild hypertension. Her mother died of breast cancer at age 59 and a cousin on her mother’s side died of ovarian cancer at age 65.
A CT scan showed small-volume ascites and bilateral 8-cm adnexal masses. Her cancer antigen 125 (CA-125) level was 285 U/mL. She underwent exploratory laparotomy followed by omentectomy, bilateral salpingo-oophorectomy, pelvic lymph node dissection, appendectomy, and resection of pelvic nodules for stage IIIC high-grade serous ovarian cancer. She had gross residual disease (2 cm) after surgery. Germline molecular testing showed she was BRCA wild type, and Myriad myChoice CDx showed homologous recombination deficiency (HRD).
She was treated with intravenous carboplatin and paclitaxel with NK1, 5HT3, and dexamethasone chemotherapy-induced nausea and vomiting prophylaxis. She experienced persistent daily nausea, with vomiting on day 1 after chemotherapy. After completion of chemotherapy, CA-125 was 14.2 U/mL; clinically, there was no evidence of disease. The patient reports continuing daily nausea.
MONK: Which adverse events [AEs] have you seen with PARP inhibitors? What do you find most challenging?
LUU: I had a patient on niraparib [Zejula] maintenance. She recurred 3 or 4 times. She’s wild type across the board and couldn’t handle 200 mg of niraparib. She had grade 3 fatigue, and we just stopped it.
MONK: There is a 100-mg dose.2
Some patients need to go to that [dose reduction]. With fatigue, I probably would not switch to another PARP inhibitor. That’s what people always think. If it was thrombocytopenia, there might be less with another PARP inhibitor.
ARJUNAN: I’ve had a pretty good tolerance. I’ve done the niraparib at [the lower-weight individualized dose] to start with. Usually, what I [find]—and I extrapolate this from experience with tyrosine kinase inhibitors—is when you go on these oral drugs, I just bring them in more frequently when they first start the treatment. I find that decreases anxiety about the treatment and the AEs, and I can monitor them quite closely and make adjustments. If I can get them through the first few weeks, I have good [adherence] with the treatment.
MONK: Because niraparib does have thrombocytopenia, [the prescribing information suggests] to do a complete blood count every week for the first month.2 They recommend that if the platelet count is less than 100,000/μL, stop [treatment] and see where the nadir goes, because it may be 90,000/μL, then you…check in a week and it plummets. Generally, we say if it gets less than 100,000/μL, stop and see what happens. If it coasts into an 80,000/μL to 90,000/μL nadir, then you can maybe start up at the full dose, but if the nadir is below 75,000/μL, you need the dose reduced. What we’re trying to minimize is grade 4 thrombocytopenia.
MANGAT: I had a patient with the BRCA mutation, and she had a response after neoadjuvant chemotherapy surgery. She was getting bevacizumab [Avastin] with the primary treatment. I put her on olaparib [Lynparza], but she had significant anemia rather than thrombocytopenia, and I had to transfuse her once. Then I looked at the label and…they said you should modify the dose only if they require frequent transfusions.4 Just 1 time is not low enough, and I looked at her for gastrointestinal [GI] bleed because she was on bevacizumab, but that was not the case. I transfused her a couple times, then I had to modify the dose, so I think I went to 250 mg twice a day.
SIDEBAR QUESTION: What is the significance of the individualized dose that was administered to some patients in the PRIMA/ENGOT-OV26/GOG-3012 trial (NCT02655016) of niraparib (Zejula)?
MONK: [Niraparib] is the only [maintenance regimen that is] once daily, the only one approved in all-comers, and it’s the only one that’s dose optimized. We decided to individualize the dose when we were well underway in the study.1 Basically, if the patient is smaller—less than 170 lbs or 77 kg—you give them a lower dose. This idea of giving the same dose to everyone, no matter how large the patient is, is sort of irrational. A lot of these patients got the higher dose even though they [had a lower weight]. We didn’t do that [as the trial continued].
The individualized dose is based on weight; it’s also based on platelet count, but almost every patient has a normal platelet count, so [generally] it’s a body weight bifurcation. If the patient is less than 170 lb, they receive 200 mg, if they’re not, they receive 300 mg.2
It’s important because if you see a smaller patient and you give the higher dose, the patient is at substantially greater risk for thrombocytopenia and [other adverse events]. If you have a smaller patient and you give [them] 300 mg, [they are reduced] to 200 mg almost right away, so there’s no dose intensity because [they] can’t take the 300 mg. So it really was an epiphany, and I applaud the FDA’s passion to dose optimize.
Do you start the PARP inhibitor after the chemotherapy, if the patient responds, and when she’s fully recovered? [I would do so] when she is fully recovered, [which] generally takes 4 to 12 weeks. If I saw a patient who had a platelet count of 130,000/μL at 4 weeks, I’d give it a couple weeks, and most of those patients are going to get to 150,000/μL. It’s either [low platelet count or lower weight], but most patients who started at a 200-mg dose [did so] because of weighing less than 77 kg or 170 lbs. This was first brought to our attention by the regulatory authorities in Europe, in the platinum-sensitive setting. You can give PARP inhibitors in platinum-sensitive maintenance, and we sent that to the European regulators, and they said we had an issue here with the weight. So we amended this frontline trial, and here we are.
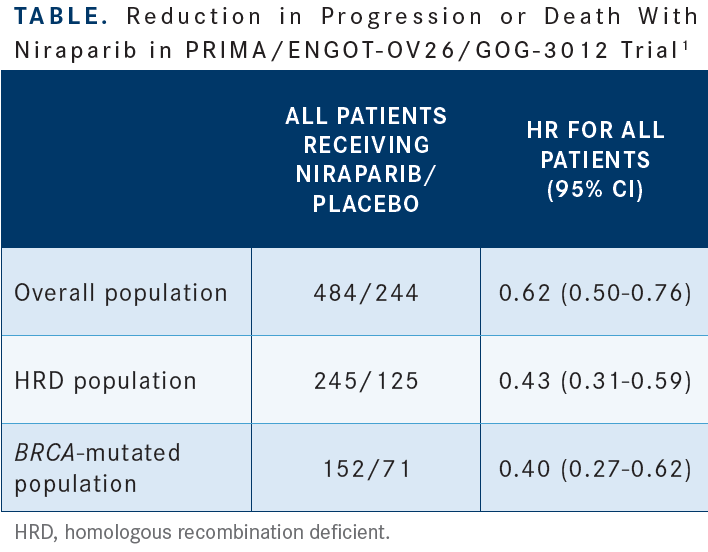
I’m excited about this because you can see the reduction in the rate of thrombocytopenia with the individualized dose of 48% to 21%, [reduction in anemia from] 36% to 23%, and a reduction [from 24% to 15%] for neutropenia [Figure 11]. Less medication is less toxic. The HR [for reduction in progression or death favoring] niraparib for patients with [homologous recombination deficiency] in the all-comers [group] was 0.43 [Table1], and [for the individualized dose], it’s 0.39. The HR in the BRCA-mutated group was 0.40 [overall, but for individualized dosing], it’s 0.29. The HR for all-comers was 0.62, and [for those who received the individualized dose], it’s 0.68. It adds confidence, and that’s why the FDA approved the individualized starting dose,3 because there’s an improvement in toxicity and no detriment in efficacy, [which is] dose optimization.
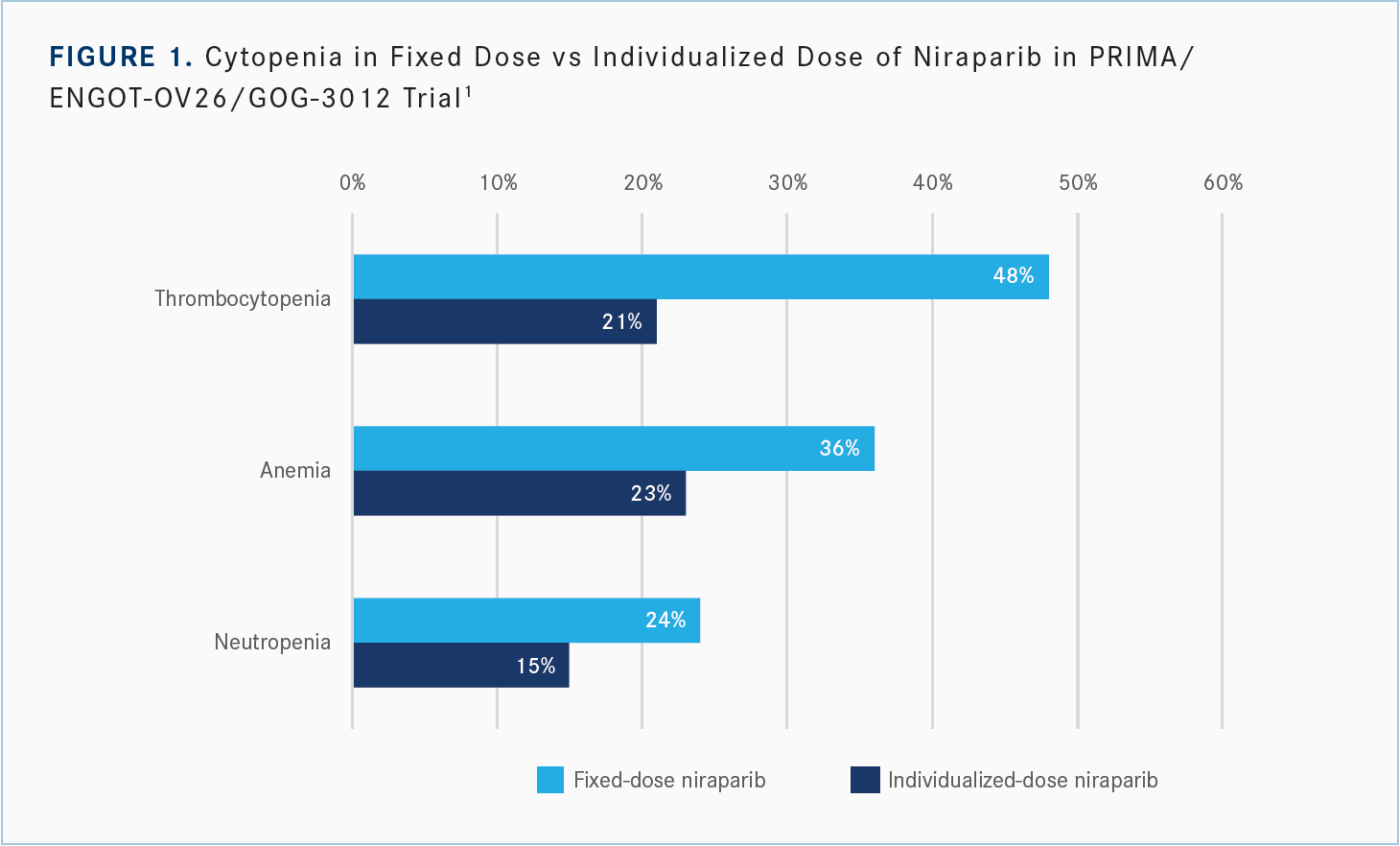
MONK: [Approximately] a third of participants have [used the niraparib individualized starting dose, which is] good. Those of you [who] have not, it may be the most important thing. Niraparib is the only once-daily [maintenance therapy], the only one that’s biomarker agnostic, and the only one that has the optimized dose.
DISCUSSION QUESTIONS
- What adverse events do you observe most frequently when using a PARP inhibitor in the primary maintenance setting?
- Which are the most challenging?
- Have you noticed any toxicities you did not anticipate/were not reported in literature?
MONK: [In my practice], we start all our patients on a prenatal vitamin just to take the nutritional anemia off the table. To your point, 1 blood transfusion may not be enough, but once you start getting into [multiple transfusions], it’s exactly as you said. Bevacizumab has a low incidence of bleeding, [but] you eliminated that, and you’re right. Olaparib can be reduced from 300 mg [twice daily] to 250 mg, then 200 mg.
MANGAT: I had another patient with prostate cancer. He had the ATM mutation, and olaparib was approved in that indication,4 [so] I gave him olaparib, too. I’ve been transfusing him more often than I would like. It’s becoming a big problem with these PARP inhibitors for me.
MONK: In a patient with prostate cancer who has had some pelvic radiation in their bone marrow, that could affect it. How did your patient with a BRCA mutation do on olaparib?
MANGAT: She is still in remission.
MONK: To my point, you can’t feel defeated because you have to reduce the dose. Congratulations.
SOLANKI: In the breast cancer trial of adjuvant olaparib in patients with the BRCA mutation [OlympiA; NCT02032823], anemia was a significant problem.5 Now these are younger patients, so it is not simply a bone marrow problem. I don’t understand the mechanism of it, because one of the comments I heard from the investigator was that sometimes the anemia was very rapid, as if you had shut off the bone marrow completely, and patients require transfusions. These are all managed by oncologists, so I don’t think the hematology was investigated in any great detail, but that has been an issue.
The other issue is the amount of dysplasia. There has been [research] that looked at why some [patients] get marrow dysplasia, and there is at least some indication that some of these patients have [clonal hematopoiesis of indeterminate potential].6 This is a fairly common occurrence in patients in their 60s and 70s, and there was a suggestion in this study that might be a factor in a proportion of patients who do get myelodysplasia…and it just happened as a result. My experience in dealing with the GI toxicity was always the worst, not because they were vomiting, [but] because they were nauseous most of the time, and we don’t know how to deal with nausea.
MONK: First of all, there’s a lot that’s unknown about the anemia, just [as] you said. The other thing with these molecular markers for acute myeloid leukemia [AML] and myelodysplastic syndrome [MDS] is they get platinum-based [chemotherapy], so [the risk of AML or MDS as an AE] is not just PARP inhibitor related. But you’re right, there are some predisposing molecular markers. Again, it’s OK to dose reduce if you have an anemia issue.
The best way to deal with nausea is to give niraparib because it’s once daily. My sense is that the nausea associated with PARP inhibitors is more irritation rather than chemoreceptor trigger zone, so…you can clear it from the upper GI through absorption. The dose is lower during the day than it is at night, [so] give niraparib at night with a small meal at bedtime and with a sedative, such as olanzapine [Zyprexa], so it’s antiemetic [and] sedating.
Niraparib has some idiosyncratic hypertension, less than 10%,2 so you have to check the blood pressure as you go. Sometimes you have to add an antihypertensive, but the jitteriness and insomnia can also happen because of this adrenergic reuptake inhibition. If I have a patient who is nauseated, I make sure they are taking their niraparib at night with a small meal and with 5 mg of olanzapine—sometimes 2.5 mg when [they are] a little groggy—but that has been pivotal for me.
REDDY: I had a patient with ovarian cancer after carboplatin, paclitaxel, and bevacizumab who I started on olaparib, but even if with a few dose adjustments, they could not tolerate it—to the extent that they had to stop it. More than cytopenia, it was [also fatigue]. She was in her late 70s, or close to 80. I started back on bevacizumab as maintenance. I don’t know [whether] it’s truly indicated, but that’s what I [gave] her. She had a BRCA mutation.
MONK: There is a randomized phase 3 trial of bevacizumab after bevacizumab in Lancet Oncology, so there is an opportunity.7 I think what you did was very smart. The discontinuation rates of all these PARP inhibitors [are] between 10% and 15%…but there is a clinical benefit, and I’m glad we could highlight this.1,8
REDDY: The benefit of bevacizumab in [patients with] BRCA is probably not as good as BRCA [with] PARP inhibitors, but that’s better than not giving it.
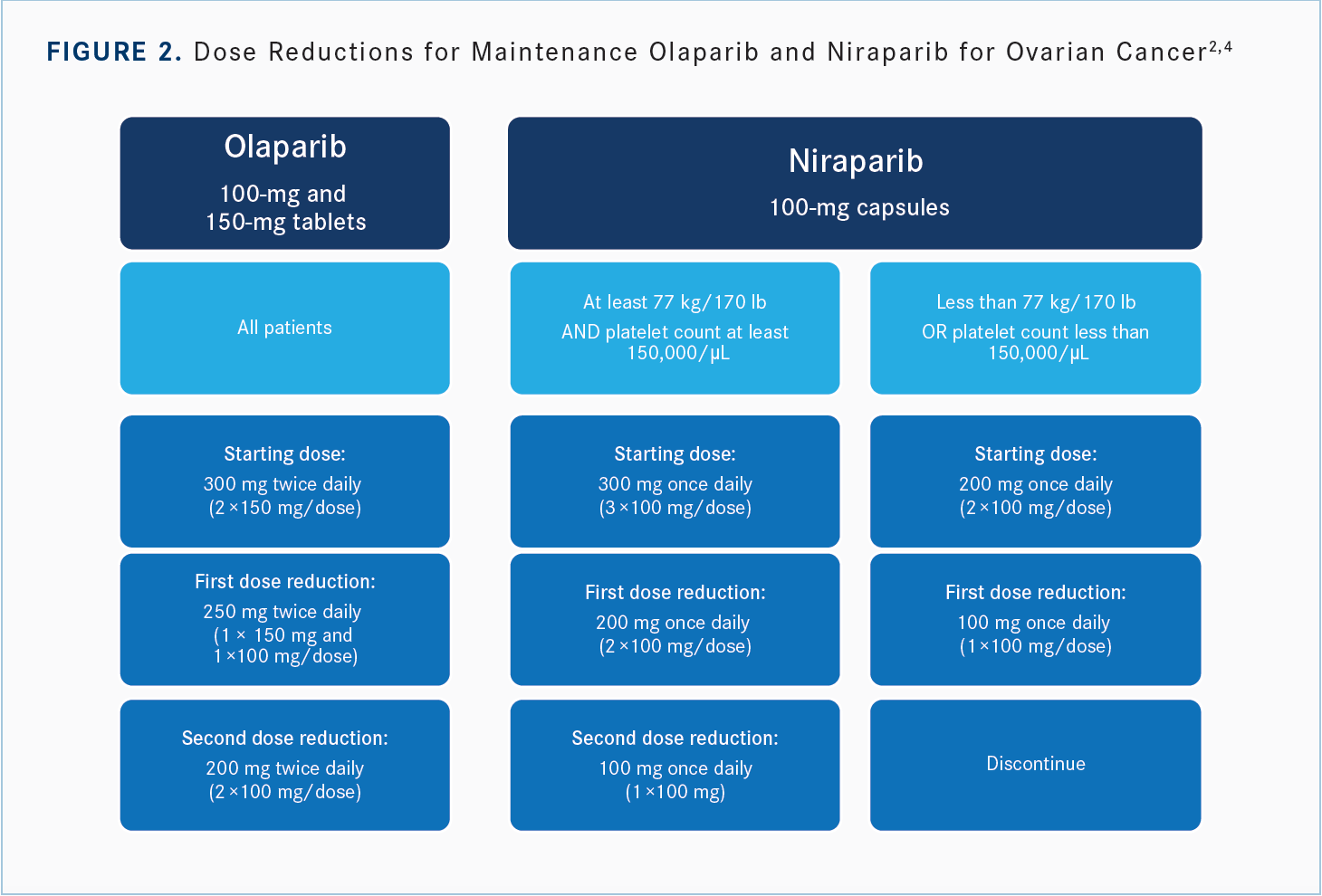
MONK: [The recommended dose reductions] for olaparib [are— as Dr Mangat said], he started at 300 mg, went to 250 mg, and things got better, so that’s what we do [Figure 22,4]. If there’s an AE, stop and recover, give them a week, which is] how long it takes to come and see me anyway. [When] a patient calls and says they have nausea, they did just what I said and it’s not working, [when I see them 1 week later, they usually say], “I stopped [taking] it, and I’m fine.” If they were taking it and were nauseated and now they’re not taking it and [aren’t] nauseated, then] that’s an easy solution, to dose reduce. [For niraparib], it’s 300 mg, 200 mg, then 100 mg if you started at 300 mg; or 200 mg, then 100 mg if you [started at 200 mg].
REFERENCES
1. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi:10.1056/NEJMoa1910962
2. Zejula. Prescribing information. GlaxoSmithKline; 2020. Accessed October 3, 2022. https://bit.ly/3M038IO
3. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. FDA. April 29, 2020. Accessed October 3, 2022. https://bit.ly/3UYgh9f
4. Lynparza. Prescribing information. AstraZeneca; 2020. Accessed October 3, 2022. https://bit.ly/3rtSKzH
5. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394-2405. doi:10.1056/NEJMoa2105215
6. Navitski A, Al-Rawi DH, Liu Y, et al. Baseline risk of hematologic malignancy at initiation of frontline PARP inhibitor maintenance for BRCA1/2-associated ovarian cancer. Gynecol Oncol Rep. 2021;38:100873. doi:10.1016/j.gore.2021.100873
7. Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928-936. doi:10.1016/S1470-2045(15)00086-8
8. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495- 2505. doi:10.1056/NEJMoa1810858

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More