Clinical Commentary: Data to Consider When Sequencing Therapy in Castration-Resistant Prostate Cancer
At a live event discussing the case of a man, aged 75 years, with metastatic castration-resistant prostate cancer, Rana McKay, MD, focused on the makeup and results of the pivotal phase 4 CARD and phase 3 VISION studies.
Rana McKay, MD
Associate Professor of Medicine
UC San Diego Health
La Jolla, CA

LIVE EVENT SUMMARY
At a live event discussing the case of a man, aged 75 years, with metastatic castration-resistant prostate cancer (CRPC), Rana McKay, MD, focused on the makeup and results of the pivotal phase 4 CARD (NCT02485691) and phase 3 VISION (NCT03511664) studies. These studies make up the basis for the use of cabazitaxel (Jevtana) and then subsequently lutetium in certain patients with prostate cancer. McKay discussed the results, challenges, and how best to sequence the therapies in a clinician’s practice.
Discussing the CARD Trial
CARD tested cabazitaxel compared with the alternate [androgen receptor] AR-targeted therapy [of either abiraterone (Zytiga) plus prednisone or enzalutamide (Xtandi)] for patients with metastatic CRPC.1 This study enrolled patients who had progressed on an AR-targeted therapy [ARTT] within 12 months of starting that drug. It’s not necessarily everybody who’s on a novel hormonal agent, but those [who] have sort of progressed, whether it was before or after [the use of] docetaxel.
The trial randomly assigned patients 1:1 to receive 25 mg/m2 of cabazitaxel, with granulocyte colony-stimulating factor [G-CSF] support, once every 3 weeks. The study was done in Europe in the post-STAMPEDE [NCT00268476] era where the frontline landscape for metastatic hormone-sensitive disease was. There was a lot of uptake of docetaxel for hormone-sensitive use in Europe when the trial was conducted, and the median duration of the ARTT use was around 7 to 8 months.
The primary end point [of the study] was imaging-based progression-free survival [PFS], which is because they used both RECIST and Prostate Cancer Working Group 3 criteria to define bone disease progression in patients. Secondary end points looked at overall survival [OS], PSA [prostate-specific antigen] response, tumor response, and other quality-of-life end points such as pain response, time to symptomatic skeletal-related events, safety, and the identification of various biomarkers as well.
When we think about their docetaxel and prior ARTT status, whether it was abiraterone or enzalutamide, there’s about an even split between the arms. [For patients given cabazitaxel,] 43.4% had prior abiraterone and 55.8% were on enzalutamide, with this similar split reflected in the comparison arm, [being the arm with patients on either abiraterone or enzalutamide]. Most of these patients received ARTTs after docetaxel.
Ultimately, the trial was a positive study that demonstrated an improvement in imaging-based PFS with cabazitaxel over the use of abiraterone or enzalutamide. The median PFS in patients given cabazitaxel was 8 months [range, 5.7-9.2] vs 3.7 months [range, 2.8-5.1] for patients given an androgen-signaling targeted inhibitor, with an [HR] for progression or death of 0.54 [95% CI, 0.40-0.73; P < .001], which was statistically significant.
Outcomes and Safety Groups in CARD
This trial did enroll patients who had an ECOG performance score of 2, and looking at time from initiation of the ARTT to disease progression, whether it was greater than or less than 6 months or it was before or after the docetaxel, across all these subgroups the direction of the HR was 0.8 toward the use of cabazitaxel.
However, I want to [reiterate that the] point of these preplanned subgroup analyses is that they were not necessarily statistically significant for every [subgroup]. The study is not powered to demonstrate significance for each of these small subgroups, but what you would want to look at is the point estimate. You want to look [to see if the] HR is trending towards cabazitaxel, and that’s what [is highlighted in this study].
Now, either radiologic or symptomatic progression or death is a little bit different than the primary end point, which was imaging based. This basically considers clinical progression in addition to radiographic progression, but there was still a statistically significant improvement with cabazitaxel vs the alternate therapy. The median PFS was 4.4 months [95% CI, 3.6-5.4] in the cabazitaxel arm compared with 2.7 months [95% CI, 2.4-2.8] in the abiraterone or enzalutamide arm, with an [HR] of 0.52 [95% CI, 0.40-0.68; P < .0001].
While OS was a secondary end point, there was a significant improvement with the use of cabazitaxel over abiraterone or enzalutamide. Median OS was 13.6 months [95% CI, 11.5-17.5] in the study arm compared with 11 months [95% CI, 9.2-12.9] for patients on an androgen-signaling targeted inhibitor, which was statistically significant with an [HR] of 0.64 [95% CI, 0.46- 0.89; P = .008]. There was also a statistically significant improvement in PSA response, objective tumor response, and pain response for patients on cabazitaxel over the hormonal agent [Figure2]. Additionally, time to symptomatic skeletal-related event was statistically significant with use of cabazitaxel, [whereas] the median time to event was not reached [95% CI, 20-NR], compared to a median of 15.7 months [95% CI, 10.8-NR] for patients on either of the other inhibitors [HR, 0.59; 95% CI, 0.35-1.01; P = .05].
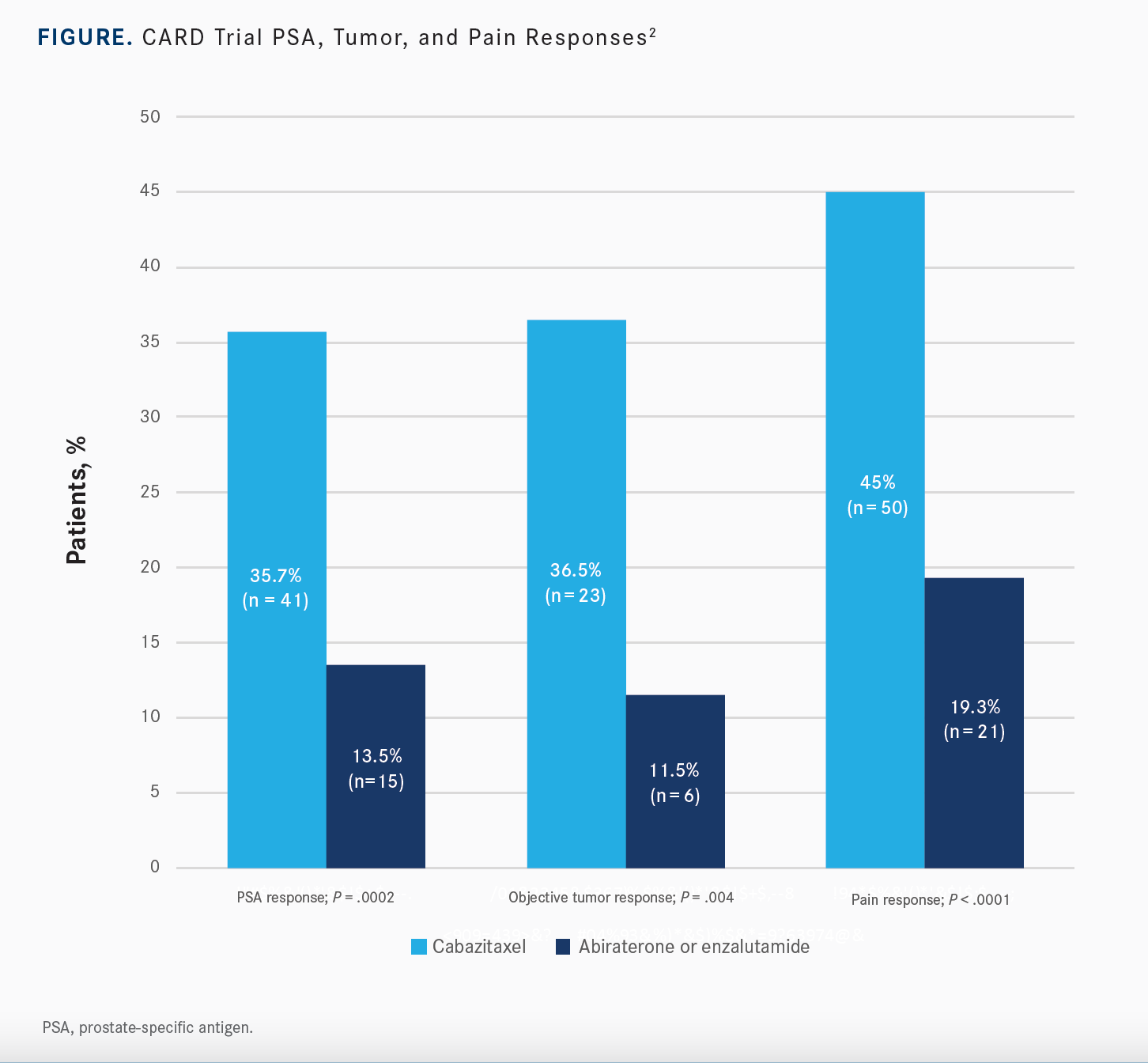
[In terms of safety,] grade 3 or greater adverse events [AEs] of any grade were similar between the 2 arms.2 It was 56% with cabazitaxel and 52% with abiraterone and enzalutamide. Serious AEs were about the same between both arms. I think what’s important to highlight is the AEs that are leading to treatment discontinuation. There are more AEs, almost a doubling of the AEs, leading to treatment discontinuation with cabazitaxel at 19.8% compared to abiraterone or enzalutamide at 8.9%. However, there is a greater likelihood of deterioration of quality of life at 3 months with abiraterone or enzalutamide.
These [patients will] have pain that’s likely related to their disease. When you give them an effective therapy and control their disease-related symptoms, they feel better. Therefore, though this is a trial of chemotherapy, we’re seeing improvements in quality of life because [most] of the patients coming into this trial are a large portion of patients who have pain.
Comparing VISION With Current Standard of Care
[VISION] is a phase 3 open-label study of protocol-permitted standard of care plus 177Lutetium [Lu]-PSMA-617 for patients with prostate-specific membrane antigen [PSMA]-positive metastatic CRPC.3 Eligible patients needed to have received both 1 or 2 lines of a prior taxane or 1 or more lines of a pathway inhibitor. Patients were allowed to receive protocol-permitted standard of care, but because this was lutetium, [the study] excluded patients given concurrent chemotherapy, immunotherapy, radium, or investigational drugs. It limited the standard-of-care arm to hormonal therapies, steroids, radiation, those sorts of things.
[Patients had to have a] performance status of 0 to 2 and they had to have a life expectancy greater than 6 months. They did have to have PSMA-positive disease based off gallium 68 PSMA imaging. This was in reference to the liver, as opposed to in reference to uptake in the salivary glands. It did not mandate [18F-fludeoxyglucose] FDG PET, as was done in the prior therapy trial, which mandated both PSMA and FDG PET imaging. Patients were randomized 2:1 to receive lutetium PSMA plus standard of care, which was given intravenously every 6 weeks for up to a maximum of 6 cycles vs just protocol-permitted standard of care. There was no crossover that was allowed, and the primary end point for the study was OS and radiographic PFS [rPFS].
When the study was first started, there seemed to be a lot of dropouts in the control arm, which obviously is going to affect the interpretation of the primary end point of PFS. After recognizing that, [the researchers] implemented protocol measures to ensure that wasn’t happening, like education to the primary investigators, education to the sites, and education to the patient that was not permitted.
There were 2 analysis data sets, 1 analysis data set after the implementation of these measures to prevent [individuals] dropping off the control arm and making the treatment arm look better. That’s sort of the analysis set for imaging-based PFS, which included 581 patients. Then all patients underwent randomization.
To give a little bit of background around why there’s this imaging-based PFS…the data post implementation of measures is to prevent dropout from the control arm. There was a fair bit of patients [who] had lung and liver metastases that were enrolled [in] the trial at around 10% for lung and just over 10% for liver. So, 97% [of patients in the PFS set] and 96% of patients [in the randomization set] had received prior docetaxel and about 40% in both sets of patients had received prior cabazitaxel.
Outcomes of VISION
There was a statistically significant improvement of rPFS from 8.7 months in the 177Lu-PSMA-617 arm compared with 3.4 months in the standard of care alone arm with an HR of 0.40 [99.2% CI, 0.29-0.57; P < .001] that is consistent with what is seen for all randomized patients. The OS data show a median OS of 15.3 months in the study arm vs 11.3 months in the control arm [HR, 0.62; 95% CI, 0.52- 0.74; P < .001]. This was a positive trial for both OS and rPFS in the rPFS analysis and overall data set.
The [main treatment-emergent AE to look at with this agent] is bone marrow suppression, certainly more than in the control. In all grades, 47.4% of patients in the 177Lu-PSMA-617 arm experienced bone marrow suppression compared with 23.4% experiencing grade 3 to 5 bone marrow suppression. A fair bit of anemia and thrombocytopenia [were] also seen while dry mouth is also commonly observed. Most of the time this tends to be low grade, but around 40% dry mouth and 40% nausea and vomiting [were] seen in the study arm. [There was also a] slight increased risk of some renal toxicity, but again, most of that is low grade that’s seen.
Based on the results of the VISION trial, the FDA approved lutetium PSMA for use in patients who had received a prior AR-targeting agent and prior taxane chemotherapy, but patients needed to have positive PSMA PET imaging to be selected for treatment.4
Administering PSMA Tests
In most institutions, the medical oncologist is not the one administering the treatment, just like radium, because of the radiation credentialing and radiation safety steps that need to happen. That either happens through nuclear medicine or radiation oncology has done some of the radiopharmaceutical and radioligand therapy.
In a lot of institutions, there’s a consult for whichever department is administering, whether it’s nuclear medicine or radiation oncology. Then they see that provider and they get the drug ordered. Regarding the imaging, that’s ordered just like any PET scan, but we’ve got [18F-DCFPyl]
PyL PSMA imaging at our institution, so we’re able to order it internally. [For other institutions that may not have that,] there are external imaging groups or other places that do offer PSMA imaging, whether it be PyL with gallium 68 PSMA-11, or PyL with F18, or whether it be the Telix product with gallium 68.
At the beginning, hopefully [individuals] align with the guidelines, get comfortable with using it in the right context. There are a lot of clinical trials that are looking at testing lutetium pre chemotherapy and lutetium for patients with hormone-sensitive disease.
The Use of Lutetium After Radium
I do think that you can give lutetium after radium because it’s a totally different kind of mechanism of action. Radium is an Α emitter that’s targeting the bone only, [whereas] Lu is a Β emitter that’s targeting PSMA cells. They may be in the bone, or they may be out of the bone, but they work differently, and I think, complementary [as well]. There are studies that are testing actinium, which is an Α particle PSMA-targeted therapy and testing the combination of both the Α and the Β particle. The biggest thing that worries me [about this approach] is the myelosuppression, just like the bone marrow toxicity.
[I do believe that] there still is a role for chemotherapy in the context for patients who do not have PSMA-expressing disease or have lesions that are seen on CT imaging that are not PSMA avid. For these [individuals who] don’t have the biomarker, or they don’t have PSMA-expressing cells, or maybe their liver lesions are non-PSMA expressing but their bone lesions are, I’m not going to be jumping through hoops to give that person lutetium right away anyway. I think in that context, it may not necessarily be the greatest option. If they’re PSMA expressing, I don’t think that if they have lung or liver or bone cancer, that will preclude one or the other. Similarly, for the high volume or rapidly progressive disease, we’ve seen that it’s an effective agent.
REFERENCES
1. de Wit R, de Bono J, Sternberg CN, et al; CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. doi:10.1056/NEJMoa1911206
2. Fizazi K, Kramer G, Eymard JC, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. 2020;21(11):1513-1525. doi:10.1016/S1470-2045(20)30449-6
3. Sartor O, de Bono J, Chi KN, et al; VISION Investigators. Lutetium-177- PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
4. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. FDA. March 23, 2022. Accessed October 1, 2022. https://bit.ly/3SRuWBq

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More