Roundtable Discussion: Selecting a Novel Hormonal Agent for Relapsed nmCRPC
During a Targeted Oncology case-based event, Alan H. Bryce, MD, discussed decision-making for treating a patient with non-metastatic castration-resistant prostate cancer.

Alan H. Bryce, MD (Moderator)
Medical Director, Genomic Oncology Clinic
Associate Professor of Medicine
Chair, Division of Hematology/ Oncology, Department of Internal Medicine
Mayo Clinic Arizona
Phoenix, AZ


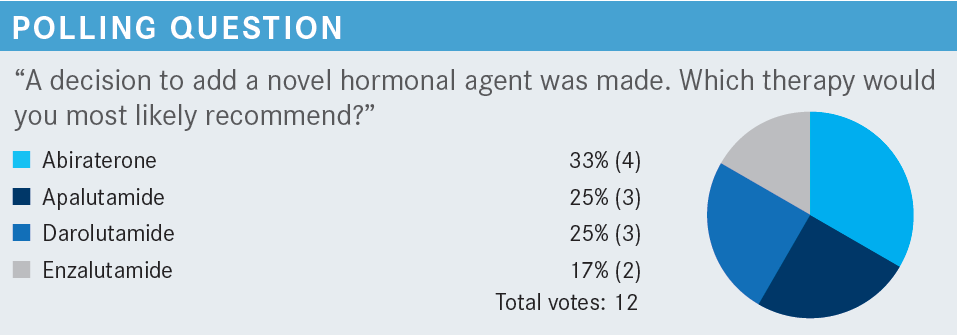
HAO: I chose darolutamide [Nubeqa] because this is nmCRPC [nonmetastatic castration-resistant prostate cancer] with biomedical relapse. The PSA doubling time was less than 10 months. Apalutamide [Erleada], darolutamide, and enzalutamide [Xtandi] are approved by the FDA [in this setting]. The patient has a seizure history, so [enzalutamide] is not preferred.1 Darolutamide has less penetration, so that is why I chose it.2
BRYCE: Absolutely. The 3 on-label drugs are the antiandrogens apalutamide, darolutamide, and enzalutamide. The [seizure] risk is an important consideration. Of these 3, darolutamide is the one that doesn’t appear to raise the seizure risk. So yes, I think that’s very appropriate.

CHERINGTON: [The goal] is always to improve survival, and [the patient is] probably not very symptomatic, so quality of life is the second thing I think about. So the question is: What is the best way to improve survival and quality of life? Do you treat right away, or do you hold off?
BRYCE: How would you counsel the patient through these options?
CHERINGTON: I try to weigh the pluses and minuses such as the potential adverse effects [AEs] of treating, whether they have a quicker or slower [PSA] doubling time, and then [I] try to do educated guesswork about whether they will benefit from starting therapy earlier and possibly dealing with AEs or holding [off on] treatment. Most of the time, patients seem to favor starting treatment sooner than later.
BRYCE: I agree.

KAHN: A lot of times when you have choices, you must go through each of them, and patients sometimes bring in their own biases. Some patients don’t like taking prednisone [with abiraterone] because it’s an extra step. If there is a history of seizures, that is something that one wants to consider as well. There are a lot of times where there isn’t necessarily a right or wrong answer. It’s more like the best one based on those factors as well as patient preferences, and unfortunately, the cost and insurance or formularies play a large role in that.
BRYCE: Does the patient’s history of seizures affect your choice?
KAHN: Well, that’s where darolutamide comes in. I think that’s OK for [patients] with a history of seizures.
BRYCE: Fair enough. There is an advantage in terms of not raising the seizure [threshold]. How do you incorporate the PSA doubling time? For example, if it were 15 months [rather than 8 months]?
SUD: The NCCN [National Comprehensive Cancer Network] guidelines state doubling time as more than 10 months or less than 10 months [to determine whether to use novel hormonal agents].3 I think I would do a symptomatic evaluation of the patient, trying to see how the PSA [level] trajectory is going forward. I am not sure whether I would be a stickler for 15 months or 10 months if the patient were symptomatic or had documented metastasis. But based strictly on the NCCN guidelines, it would be a cutoff of 10 months.
BRYCE: The data behind that are based upon the fact that all the clinical trials for FDA approval had a 10-month [minimum] doubling time as part of the eligibility criteria. That [doubling time] was derived from [data from] Matthew R. Smith, MD, PhD, showing the risk of metastatic disease starts to go up.4 There were inflection points right around 10 months, so that’s how it came to be. We don’t think 10.5 months is that different [from 10 months, but] we must have a consensus, so that’s the way the trials were developed. What comorbidities and considerations about age and frailty influence your selection?
SMITH: Abiraterone has a warning about fluid overload and heart failure.5 So that is something you will see. As we talked about, there are seizures. Darolutamide seems to be extremely well tolerated except by insurance companies. They usually have a formulary, and they want [a patient] to try something else like enzalutamide first. But darolutamide or apalutamide look to be the best tolerated. Abiraterone has some real risks in some [individuals] with comorbidities. They are getting prednisone twice daily, and [there is the risk of] heart failure.

ROZELL: We should try not to do cross-trial comparisons. A lot of times, I still look at the AE profile and insurance preference. Fatigue is a huge problem for my patients. I am not sure whether you have any [advice] on how to deal with fatigue, but that often causes dose reductions.
BRYCE: We talked about this when enzalutamide first came out. Patients can get profound asthenia with enzalutamide, and that biased us at the Mayo Clinic [Arizona] more toward abiraterone. I believe it is because of the activity against the CNS [central nervous system] androgen receptor. This is where we see an advantage with darolutamide [for] that overall fatigue. Over the years, methods have been tried like steroids and dose reductions, but they don’t seem to make much difference, at least in my experience. We’ve had patients in whom we intentionally stopped enzalutamide because of asthenia, and 4 weeks later they say, “I didn’t know I could feel this much better.”
I do probably use darolutamide more than enzalutamide because of that, and I see less fatigue as a result. The thing I would say about the OS and MFS, to refine an earlier point about first-generation antiandrogens, is we don’t have that level of OS data for them, and that’s the key difference. It doesn’t mean that you can’t go from first-generation agents to the next-generation ones and still get the results.
ROZELL: A lot of the AEs are similar [among these agents]. I can’t say that I’ve had any experience with seizures [in my patients] or that sort of thing. I usually see hypertension, and I see a lot of fatigue, dizziness, and falls among them.
SORIANO: Well, first is being able to do it. Where I am in Sterling, Colorado, we don’t have the PSMA PET scan. All we do are fluciclovine PET scans. I think the PSMA [PET scan] results would change my opinion about treating [patients] more aggressively, especially if they have metastatic disease found on the PSMA PET scan.
BRYCE: [For those] who may have access to PSMA imaging, how do you conceive this should alter your approach in this space? Does anybody have access to PSMA [imaging], and have you been getting it for these patients?
SHELANSKI: I haven’t, but you said that 98% of these patients are going to have a positive PSMA result. I guess the real question is, if we have someone who has low-volume metastatic CRPC is the treatment approach any different than if they had recurrent nmCRPC? If we look at PSMA [levels], we can say most of these patients [have metastases]. We just don’t have the imaging modality to demonstrate it, so it wouldn't change my approach.
BRYCE: I would say the only difference is that with current FDA labeling, darolutamide is available [for] nmCRPC, but not mCRPC.6 It is a silly distinction because I agree with your point. It’s not a biologic distinction; it’s just about how [detailed the scan is]. But [it could cause issues with] insurance companies and peer-to-peer reviewers. If you say you want one drug, they [focus on] this detail. Most [oncologists] aren’t ordering a lot of PSMA scans yet. This is a question we will revisit over time, as PSMA scans get more common.

MUSHTAQ: I would say [the differences] are based on the AE profile, because in terms of efficacy, I don’t want to compare the SPARTAN [NCT01946204], PROSPER [NCT02003924], and ARAMIS [NCT02200614] trials. But in terms of HR, I think apalutamide and enzalutamide had the best HR for metastases, at about 0.3 compared with 0.41 for darolutamide [From the Data7-9]. But the AE profile was much better with darolutamide, including less fatigue, which was almost less by half, and high blood pressure incidence was a quarter of what apalutamide had. There were fewer chances of seizures, almost [none].7-9 So because of that overall AE profile and considering these are mostly older patients, I would prefer darolutamide. But if I have somebody who is fit and I don’t have to worry about the AEs, I could probably choose apalutamide too. But I’d want to make sure I document that there are no PSMA-positive metastases, which is a challenging thing because we don’t have PSMA scanning now.

DOMENECH: I have used apalutamide. I have seen the rash. I think we contraindicated enzalutamide knowing the patient had a seizure disorder. I also had a problem with [patients experiencing] diarrhea where I had to reduce the dose. I haven’t used darolutamide. I will consider it next.
AMARANENI: I have used enzalutamide and apalutamide in this setting. Both seemed to be similar in terms of AEs in my patients. None of them had seizures, which I know is more common in the enzalutamide population. But both groups experienced significant fatigue, and that seemed to be most of what they complained about.
In terms of how long they typically received [one of these agents], I believe one of my patients was on it for about 2 years. I think it was the patient on enzalutamide. The patient on apalutamide remains on it. The AEs seem to be stable through the time that I’ve used [these agents]. I haven’t seen patients feel worse as they’ve stayed on them. It’s just that they start them, develop the fatigue, and then, at least when I’ve used [these agents] for metastatic patients, they either say it’s intolerable or handle it.
REFERENCES
Xtandi. Prescribing information. Pfizer; 2018. Accessed October 4, 2022. https://bit.ly/2Vo2VVb
Crawford ED, Stanton W, Mandair D. Darolutamide: an evidenced-based review of its efficacy and safety in the treatment of prostate cancer. Cancer Manag Res. 2020;12:5667-5676. doi:10.2147/CMAR.S227583
NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 1.2023. Accessed September 26, 2022. https://bit.ly/34xiIXZ
Smith MR, Saad F, Oudard S, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31(30):3800-3806. doi:10.1200/JCO.2012.44.6716
Zytiga. Prescribing information. Janssen Biotech Inc; 2020. Accessed October 4, 2022. https://bit.ly/3RzINvg
Nubeqa. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; 2022. Accessed October 4, 2022. https://bit.ly/3C01ane
Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(suppl 15):5516. doi:10.1200/JCO.2020.38.15_suppl.5516
Sternberg CN, Fizazi K, Saad F, et al; PROSPER Investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892
Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/ NEJMoa2001342

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More