Roundtable Discussion: Salgia Looks at Testing and Treatment for NSCLC With EGFR Exon 20 Insertions
During a Targeted Oncology case-based roundtable event, Ravi Salgia, MD, PhD, discussed with participants their approach to molecular testing and treatment of a patient diagnosed with non–small cell lung cancer.
Ravi Salgia, MD, PhD (Moderator)
Chair and Professor, Department of Medical Oncology & Therapeutics Research
City of Hope Comprehensive Cancer Center
Duarte, CA

SALGIA: What would you do in terms of this patient, now that you have all this information?
WATSON: I would arrange for a biopsy of whatever seems to be most accessible—probably the lung lesion, if possible. I would then send for NGS [next-generation sequencing].
I’m newer in practice and have just been doing the FoundationOne testing, sending that on the biopsy. I haven’t been sending much in terms of a liquid biopsy. I’m going to be looking into that more.
SALGIA: That’s great. Would you want to send for IHC [immunohistochemistry] for PD-L1 testing?
WATSON: Yes, absolutely. I would send it for PD-L1 testing as well.
FARJAMI: With EBUS-guided biopsies, we have to scan these samples. We always get these reports from the pathologists that there are not enough samples for going through all the NGS, and even sometimes IHC. This is a case where I’d like to get a liquid biopsy too.
SALGIA: That’s exactly right. It depends on where you send the tumor tissue. [It makes a huge difference] if you can have cross talk and cross collaboration with wherever you’re sending it. If you say, “It’s a scanty cytology specimen, but I’m very interested in these 11 biomarkers,” they can stack the deck in your favor. [However, they then] might [ask whether your priority is mutation or fusion]. That’s exactly why we work so closely with the pathologist. Of course, one of the other big things is you don’t want the pathologist to do a million stains on that specimen. It’s TTF1 positive, it’s adenocarcinoma by microscopy—stop. Don’t do any more IHC, except for PD-L1, the [PD-L1 IHC 22C3 pharmDx] in case, then go on to the NGS. The technology has been getting better and better.
I was at [The United States and Canadian Academy of Pathology], and I presented to pathologists on how one should make clinical decisions. Our pathologists are very cognizant that at the end of the day, our decision-making depends on molecular testing. They’re coming up with the cytology specimen. Sometimes they can do laser capture microdissection.
It is important to talk to the…pathologist [who does the EBUS] to say, “Only do the bare minimum of testing to make sure it’s an adenocarcinoma and it’s a lung cancer.” I think all you need is a TTF1 or a napsin A, but sometimes there’s a question. For example, adenocarcinoma vs squamous cell carcinoma: They might want to do a p40 [IHC] to rule out squamous cell carcinoma. Sometimes other labs do p63 [IHC], and that’s OK. Then save everything for the NGS. I agree with you about the liquid biopsy, as it is very important.
ZHOU: In my practice, I usually send the tissue to FoundationOne for PD-L1. I do run into problems with the EBUS, [as there may not be] enough sample to do the test. I usually wait until the tissue comes back. If it says [there isn’t] enough tissue, then I’ll send a liquid biopsy. Has [anyone] had a problem with insurance approval when you send them both at the same time?
CHOUDHRY: I routinely send Guardant360 and FoundationOne on all patients, and I’ve never had an issue with coverage.
ZHOU: But at the same time or sequential?
CHOUDHRY: At the same time, because I send Guardant360 right away and it comes back in 7 days.
CHARU: I have had no problems. I think Guardant themselves check with the insurances.
SALGIA: Guardant forgives a lot of those if the insurance doesn’t pay for it, but I think you should talk to the company, and they can come up with an equation for you. I think [many of] us do that. I do that in our practice, too, where we send it for molecular testing internally for an NGS called HopeSeq. But for liquid biopsy, we send it to Guardant. [Are there] any other thoughts anyone else has?
CHOUDHRY: What about HER2 mutations in lung cancer?
SALGIA: For HER2 mutations, yes. HER2 mutations can be actionable by certain drugs at times. For example, poziotinib is an interesting drug that has actionable capabilities within HER2 mutations.1 I think you have to look at the HER2 mutations, where they are, and which region. At the same time, if you have a HER2 alteration, can trastuzumab deruxtecan [Enhertu] also be effective? [However], those are not approved at this moment in time. I routinely do HER2 analysis for an amplification and mutation, then I try to see [whether] the patients can qualify for a clinical trial.
CHOUDHRY: Amplification by IHC or by molecular testing?
SALGIA: Molecular testing is what I like, but one can do IHC. There are some protocols that are developed for looking at HER2 expression by IHC…but 2+ [HER2 expression] is not as well characterized for lung cancer compared with breast cancer. I think you have to look at the clinical trials rather than off trial. We have several of them if anyone is interested.
ZHOU: Let’s say the patient had EBUS and the tissue is not enough for whatever lab you sent it to. You get a liquid biopsy. The liquid biopsy comes back as negative. Will you trust the liquid biopsy, or do you want to get more tissue? [For example], for a patient who is older, Asian, [and a] nonsmoker.
SALGIA: I tend to think about the physical emergency. Do you need to start treatment now, or can you repeat a biopsy, then send it out for molecular testing? If it’s diffuse metastatic disease, a bulky tumor, [and] the patient’s incredibly short of breath or they have [multiple] neck nodes, then I don’t think you have a choice.
Let’s say it’s an adenocarcinoma. Then I might do carboplatin and pemetrexed [Alimta]. I won’t do immunotherapy. Because for immunotherapy, if it’s a targetable lesion, it could be trouble in the future. If they get a TKI [tyrosine kinase inhibitor], then they could get into a fatal pneumonitis issue. Then if I can stabilize and the patient has some tissue we can still biopsy very easily, let’s say with CT-guided biopsy, I still may go after that. It depends on the tumor burden and the physical crisis that would help you to [decide whether to] repeat biopsy now or after 1 or 2 cycles. Or, if they respond beautifully to those 1 or 2 cycles, then just wait for the future.
CHOUDHRY: If a patient is in visceral crisis and you need to start treatment but your pretest probability is quite low— they’re a smoker and they’re going to have a mutation—do you still hold off on immunotherapy in all-comers?
SALGIA: I personally do. I tend to hold off the immunotherapy unless it’s small cell lung cancer; then we would use chemoimmunotherapy.
CHOUDHRY: Is the pneumonitis risk just an EGFR-TKI effect or is it a pan-TKI effect?
SALGIA: [Most] of the data are only in the EGFR-TKIs. You have to take it with a grain of salt. Is it going to be true for a [targeted therapy for] MET exon 14 [skipping], ALK, or ROS1? You’ll have to make that clinical decision rather than an evidence-based decision.
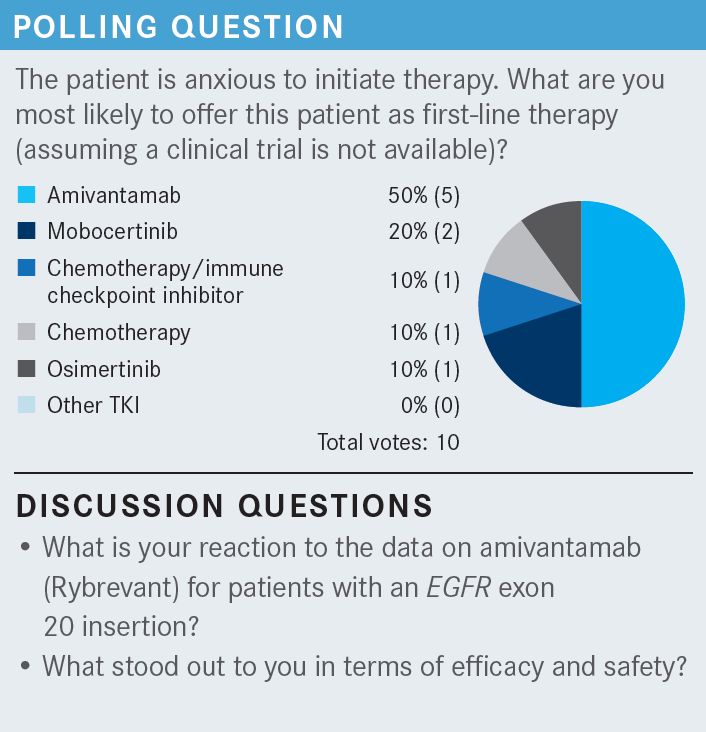
SALGIA: Has anybody else used amivantamab? What do you think [of the data]?
JANG: My impression on the data is, in general, amivantamab is probably a slightly better drug vs mobocertinib [Exkivity]—maybe a 10% to 15% better response [From the Data2,3]. It is my understanding of the infusion reaction that it typically only happens the first time. Then for the subsequent dose, there is virtually no infusion reaction. I have never given it, but that’s my impression of amivantamab.

SALGIA: We’re going to learn more about the real-world use over time, as well. That’s exactly right, based on the clinical trial [CHRYSALIS; NCT02609776].2
CHOUDHRY: I also have a patient right now. She had PD-L1 expression of 0%, so she initially got carboplatin, pemetrexed, [and] pembrolizumab [Keytruda] with good response, then she progressed on pemetrexed and pembrolizumab. She is tolerating it well, except some mild rash. She’s on her eighth cycle right now, but the response has not been that great. She had a little bit of mild progression. I’m continuing, and she tolerates it very well. Is the risk of pneumonitis the same because she already got pembrolizumab and now she’s getting amivantamab, as with the oral [EGFR inhibitor]?
SALGIA: I think so, in our experience.
CHOUDHRY: You would not have given her pembrolizumab? She had [prior] chemoimmunotherapy.
SALGIA: Did you know up front that she had the EGFR exon 20 insertion?
CHOUDHRY: I’m not sure [whether] all the information was back. I know that in the lung panel, she didn’t have the classic EGFR mutation, exon 19.
SALGIA: You [must] go with caution. [It’s] important [for all of us to realize that], from the IMpower150 study [NCT02366143] when they [investigated] the EGFR- and ALK-positive population, a quadruplet regimen has been used for the common EGFR mutation: carboplatin, [paclitaxel], atezolizumab [Tecentriq], and bevacizumab [Avastin].4 That combination works, yet it’s not giving that pneumonitis that one expects if you use osimertinib [Tagrisso] after progression, if it’s L858R.
[However], what are the true data if you use carboplatin, pemetrexed, and pembrolizumab in an EGFR mutation up front and you didn’t know the molecular testing, or it came back [quantity not sufficient]? And those data are not as well publicized or existent. People extrapolate from the Memorial Sloan Kettering [Cancer Center] data that was published to say it’s a tough thing,5 but I don’t know [whether] we have enough. I don’t think you went wrong in any way, but if we know they have a mutation, then chemotherapy followed by targeted therapy, or targeted therapy up front, is reasonable. If you don’t know, you have to make your best judgment.

SALGIA: Dr Farjami, I wonder what your thoughts are. You have had experience with both of these drugs.
FARJAMI: I picked 1 patient of mine [whom] I put in the study [of mobocertinib; NCT02716116].3 I have… concerns…about the tolerability. It’s a tough TKI that’s managed with a lot of adverse events, including rash, paronychia, diarrhea, and peripheral edema. It’s not a very easy drug to manage.
For a targeted drug that you sometimes want to use in the frontline setting, you expect [higher] response [rates] if you [compare with targeted therapies for] the other actionable mutations. The results of this study were for [patients who received prior] chemotherapy, but I think the overall response rates are [approximately] in the range of 20% to 30%. I’m wondering what your thoughts are about these response rates to a targetable mutation.
SALGIA: I referred a young woman to [Dr Farjami’s] site for these clinical trials to combine chemotherapy with these targeted agents. I think that’s the next wave, as we’ve learned. I think combining chemotherapy with TKIs or chemotherapy with immune checkpoint inhibition to enhance the progression-free survival [and] overall survival [is] where we’re going to make a huge difference in the up-front setting.
FANG: I was told that mechanism-wise, they’re against each other. The TKI is “putting the cancer to sleep,” [whereas] for chemotherapy, you want cancer [cells] to divide. Is that right?
SALGIA: Don’t forget the first studies came out of China, for example, in EGFR exon 19 deletion or in L858R, where they combined chemotherapy with gefitinib [Iressa] and were able to show a much better response rate.6 The follow-up studies came from India, [which showed] chemotherapy plus TKI worked much better.7
We also know that in certain situations—[for] example, in my clinical practice, and there are clinical trials ongoing for EGFR mutations—you can combine osimertinib with chemotherapy to get a better therapeutic response up front.8 I’m not necessarily sure I [accept] the terminology of cytostatic vs cytotoxic [as we were taught in] medical school and fellowship. I’ve asked us to [ignore] those 2 terms. Ultimately, if there’s synergism between the 2 pathways—chemotherapy and TKI—we should use that in our patients. I think that’s been shown already for chemotherapy plus TKI. [Several of] us tend to do that if you have very bulky disease with an EGFR mutation. Then you can drop the chemotherapy later.
FARJAMI: When our patients come and talk to us and we plan out our treatment plan, the paradigm, and what is approved in this setting, it’s hard for us to start with chemotherapy if you want to go by the guidelines. The FDA approval [for mobocertinib and amivantamab] is after exhaustion of the chemotherapy.9,10 Many patients are pressing; they like to start with the targeted therapies. I’m looking forward to these combinations because, as you said, I think that’s the way to go.
REFERENCES
1. Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40(7):702-709. doi:10.1200/JCO.21.01113
2. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-3402. doi:10.1200/JCO.21.00662
3. Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021;7(12):e214761. doi:10.1001/jamaoncol.2021.4761
4. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi:10.1056/NEJMoa1716948
5. Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30(5):839-844. doi:10.1093/annonc/mdz077
6. An C, Zhang J, Chu H, et al. Study of gefitinib and pemetrexed as first-line treatment in patients with advanced non-small cell lung cancer harboring EGFR mutation. Pathol Oncol Res. 2016;22(4):763-768. doi:10.1007/s12253-016-0067-4
7. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
8. Rebuzzi SE, Alfieri R, La Monica S, Minari R, Petronini PG, Tiseo M. Combination of EGFR-TKIs and chemotherapy in advanced EGFR mutated NSCLC: review of the literature and future perspectives. Crit Rev Oncol Hematol. 2020;146:102820. doi:10.1016/j.critrevonc.2019.102820
9. FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. FDA. Updated September 16, 2021. Accessed September 30, 2022. https://bit.ly/3Ebp1mN
10. FDA grants accelerated approval to amivantamab-vmjw for metastatic non-small cell lung cancer. FDA. May 21, 2021. Accessed September 30, 2022. https://bit.ly/3CnoTPA

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More