Hamid Reviews Options for Patients With Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma
During a Targeted Oncology case-based roundtable event, Omid Hamid, MD, discussed the data supporting the use of hedgehog pathway inhibitors and immunotherapies for skin cancers.

Omid Hamid, MD
Director, Melanoma Program
The Angeles Clinic and Research Institute
Cedars-Sinai
Los Angeles, CA

Targeted OncologyTM: What role do Hedgehog pathway inhibitors (HHIs) play in BCC?
HAMID: The HH pathway is mutated in about 90% of BCCs. [HH inhibits patched (PTCH1)], PTCH1 inhibits SMO [smoothened], and SMO is a stimulator of cell division and growth. By inhibiting PTCH1 or SMO, we inhibit cell growth.1
There are 2 approved SMO inhibitors: vismodegib [Erivedge] and sonidegib [Odomzo]. Vismodegib is indicated for locally advanced and metastatic [BCC], and sonidegib is approved only for locally advanced [BCC]. These drugs are supplied as single capsules and are dosed daily. Vismodegib can be administered with or without food and sonidegib should be administered 1 hour before or 2 hours after a meal.2
We have seen high response rates [with these drugs], but the adverse events [AEs] make them difficult to give. [The AEs include] muscle spasms, arthralgia, hair loss, dysgeusia— a bad taste in the mouth that leads to weight loss—anorexia, fatigue, nausea, vomiting, and increased creatinine kinase. We have some [difficulty] managing these AEs because most are not related to laboratory abnormalities.
[Offsetting these difficult AEs] are high response rates. Vismodegib has a response rate of 43% in locally advanced BCC and 30% in the metastatic setting. Sonidegib has a response rate of 44% in locally advanced BCC.3,4 The question becomes how to work with these drugs to avoid AEs. Because of the [pharmacokinetics] of these drugs, they have to be dosed daily, [with no days off], and they can’t be decreased in dosage. So most physicians give [patients] a drug holiday.
Nausea, diarrhea, constipation, and vomiting were [common] in the trials of vismodegib; [these AEs were mostly grade 1 and 2]. Fatigue of grade 3 affected 5% of the patients, [and] fatigue of all grades affected 40%. Weight loss of grade 3 affected 7% of patients, and weight loss of all grades affected 45%. There was a very high rate of muscle spasms [grade 3, 3.6%; all grades, 72%]. It is difficult to avoid muscle spasms; some [physicians recommend] hydration, stretching, or vitamins. Dysgeusia and ageusia did not occur at grades higher than grade 2 [and affected 55% and 11% of patients, respectively]. Finally, alopecia, which can be irreversible, [did not occur at a grade higher than 2 and affected 64% of patients].5
What data support the use of immunotherapy to treat BCC following HHI therapy?
Immunotherapy is indicated for use in highly mutated tumors, and that is important because BCC tumors are highly mutated. A phase 2 trial [NCT03132636] of cemiplimab-rwlc [Libtayo] examined adult patients with metastatic or locally advanced invasive BCC [who received] the approved dose of cemiplimab every 3 weeks. Tumor assessments [were conducted] by investigator review and RECIST 1.1. Patients had to have experienced prior progression, intolerance to [HHI] therapy, or no better than stable disease [SD] after 9 months. Furthermore, patients had to have at least 1 [measurable baseline] lesion. Exclusion criteria included prior treatment with anti–PD-1 or anti–PD-L1 therapy, autoimmune disease [that required systemic immunosuppression], and concurrent or recent malignancies.6
Demographically, the patients in this trial are similar to patients you see in your clinics. All had an [ECOG performance status] of 1 or 2, and most had received an HHI; 94% had received vismodegib, 6% had seen sonidegib, and 11% had changed from one to the other. The previous HHI had been discontinued because of progression in 71% of patients, and [38%] had discontinued because of drug intolerance. A small percentage [8%] discontinued prior therapy because they had achieved no better than SD after 9 months. [In 89% of the patients,] the primary BCC site was the head and neck.
At first evaluation, [49% of patients achieved] SD, [25% had] partial response [PR], and very few patients had progressive disease [11%]….Not only was the objective response rate [ORR] high at 31% [95% CI, 21%-42%], but there was clinical benefit of disease control in 80% [95% CI, 70%-88%] of the patients [Table6]. Durable disease control was experienced by 60% of the patients.
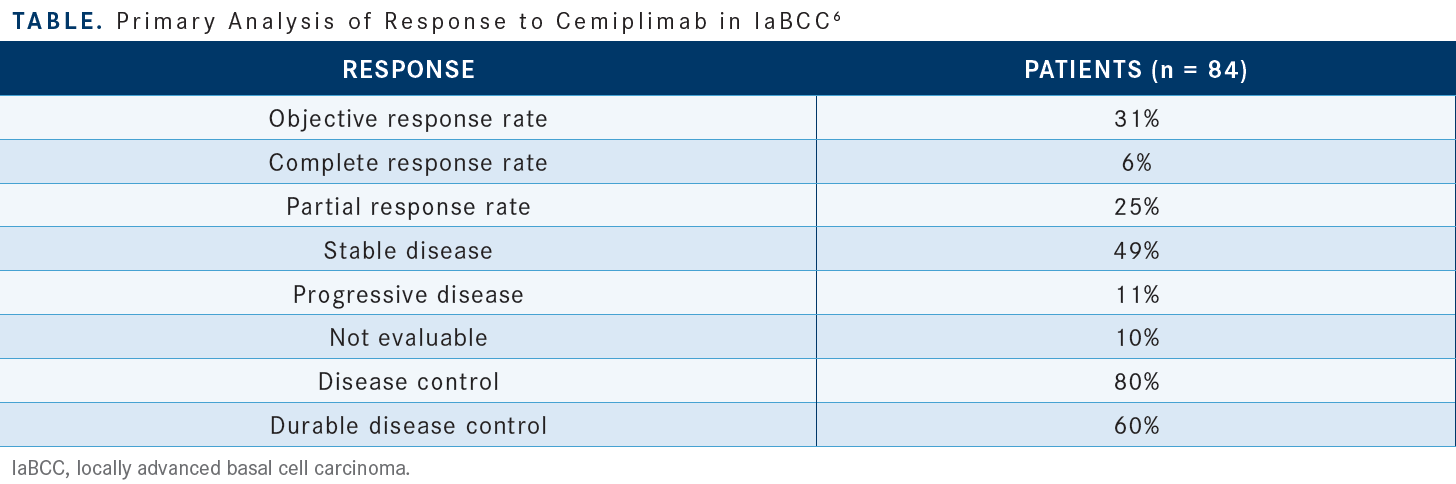
The duration of response [DOR] was greater than 6 months in [79%] of the patients and greater than 12 months in 46% of the patients. [These data are taken from a time by which] most of the patients had been evaluated. There were only 1 or 2 patients whose [response didn’t extend] past 12 months. This is an important therapy with durable benefit and a high clinical benefit rate.
The 6-month progression-free survival [PFS] was 76% and the 12-month PFS [was 57%]. The median overall survival [OS] was not reached. The 2-year OS was 80%.
[Cemiplimab provides] durable benefit with a manageable toxicity profile. This profile is similar [to those of] other anti–PD-1 checkpoint inhibitors. The most common treatment-related AEs [in this trial] included fatigue, itching, and asthenia, and the most common grade 3 [immune-related] AE [irAE] was colitis, occurring in 3 out of 84 patients. Overall, irAEs affected [25%] of the patients, and grade 3 irAEs occurred in only 10% of the patients. There were no grade 4 or 5 irAEs. [This drug was] tolerable, the toxicities were manageable, and no toxicities led to hospitalization.
The AEs that occurred in at least 10% of the patients in this study included fatigue, musculoskeletal pain, and diarrhea, [but there was no] asthenia, cramping, or changes in taste [as were observed with vismodegib].7
How do you manage HHI-associated AEs?
Minoxidil [Rogaine] can help with the alopecia. The dysgeusia and the ageusia [are caused by] neural responsiveness and change in the taste buds. [For these,] we discuss a nutrition consult, but I’ve also spent a lot of time talking directly to patients about [the importance of] eating in order to maintain their body weight and how changing their diets can help. Weight loss includes muscle loss, so activity [is also important].8
I have found the muscle spasms very difficult to manage. There are published data about calcium channel blockers such as amlodipine [used as management tools]. My patients have reported success with physical activity and stretching. Additionally, use of hydration and L-carnitine [have anecdotal support].

What do the National Comprehensive Care Network (NCCN) guidelines recommend for systemic therapy of cSCC?
Systemic therapy is not recommended for local disease amenable to curative surgery. For primary [and recurrent] locally advanced disease, if surgery is not feasible, radiation therapy [can be considered, followed by consideration of] systemic therapy. For regional disease and distant metastatic disease, options include radiation therapy, systemic therapy, or both.9
If you’re considering chemotherapy, [the purpose of the] chemotherapy is to work as a radiation sensitizer. We lack data for regimens that show survival or disease control benefit for patients with cSCC. Most of the data come from studies of head and neck SCC, which is a different beast. Keeping that in mind, the NCCN guidelines for systemic therapy alone are pembrolizumab [Keytruda] and cemiplimab, both based on clinical studies, and clinical trial.9 Some clinical trials are looking at radiation [therapy in combination with these systemic options]. At our clinics, chemotherapy is used only if you run out of other options.
The [patient we are discussing] is someone who could be considered unresectable. Even if you thought they were resectable, they could still be a good candidate for cemiplimab based on its approval in patients whose risk of recurrence you might consider to be too great and who you might think would not benefit from initial radiation therapy or surgery.
What data led to the approval of cemiplimab for the treatment of cSCC?
Those [data come from the] EMPOWER-CSCC-1 trial [NCT02760498], which examined adult patients with metastatic CSCC in groups 1 and 3 or locally advanced CSCC in group 2. [Groups 1 and 2 were treated with weight-based dosing]; group 3 received flat dosing. The key inclusion criteria for all groups included the presence of measurable disease and an ECOG score of 1 or 2. Exclusion criteria included the presence of [recent, systemically treated] autoimmune disease, prior treatment with anti–PD-1 or anti–PD-L1 therapy, [history of organ] transplant, and other malignancies.10,11
In this study, 66.3% of the patients were receiving cemiplimab as first-line therapy. The population was mostly male, and all patients had an ECOG score of 0 or 1 [44.6% and 55.4%, respectively]. The primary tumor site was head and neck in [67.9%] of the patients, and the disease was metastatic in 59.6% of the patients and locally advanced in 40.4%.12 The background for this comes from the idea that tumors with a high mutational burden respond well to immunotherapy. If you look at a cancer atlas, [you’ll find that] the tumors with the highest mutational burden are cSCCs, and [the mutational burden is increased] 10- to 100-fold in the immunosuppressed setting.
The ORRs observed in this immune checkpoint inhibitor study are some of the highest ORRs [achieved with] a single agent. In group 1, composed of patients with metastatic disease who received weight-based dosing, the ORR was 50.8% [95% CI, 37.5%-64.1%]. In group 2, composed of patients with locally advanced disease who received weight-based dosing, the ORR was 44.9% [95% CI, 33.6%-56.6%]. Finally, in group 3, composed of patients with metastatic disease who received a flat dose, the ORR was 42.9% [95% CI, 29.7%-56.8%]. The ORR of the total population of 193 patients was 46.1% [95% CI, 38.9%-53.4%].12
The complete response [CR] rates of groups 1, 2, and 3 were [20.3%, 12.8%, and 16.1%, respectively]. The disease control rates [DCRs] were 71.2% for group 1, 79.5% for group 2, and 64.3% for group 3. The durable DCR was 61.0% for group 1, 62.8% for group 2, and 57.1% for group 3. The rate of 12-month DOR was 89.5% for group 1, 83.2% for group 2, and 91.7% for group 3. The rate of 24-month DOR was 68.8% for group 1, 62.5% in group 2, and not evaluable in group 3.12 [These results contrast with our] experience with chemotherapy and EGFR-targeted therapies, which has been that, although response rates were high, the DOR was low. The results of the EMPOWER-CSCC-1 trial led to the approval of cemiplimab.13
In the New England Journal of Medicine paper [by Migden et al], there was strong photographic evidence of the power of this drug. The images showed a 62-year-old man with multifocal [disease that disappears] with what looks to be a CR, and an 83-year-old man with infiltrative disease that exhibited an ongoing PR.14
The median PFS was 18.4 months, and the 24-month PFS rate was 44.2%. The median OS was not reached, and the 24-month OS rate was 73.3%. I have found it very interesting that in immunotherapy trials, [there tends to be a progressive slowing of the rate of change in PFS and OS]. This is something that has not been seen previously.12
What are the data for pembrolizumab in this patient population?
The KEYNOTE-629 trial [NCT03284424] looked at pembrolizumab in similar cohorts of patients, with either locally advanced or relapsed metastatic CSCC. The patients received pembrolizumab at the approved 3-week weight-based dosing for 2 years prior to survival follow-up.15
At baseline, 72.3% of all patients had a PD-L1 combined positive score of at least 1, and 61.0% had a tumor proportion score of less than 50%. The primary tumor stage [was T3 in 32.1% of patients, with the remainder distributed approximately equally among stages T1, T2, T4, and TX]. From among the patients with relapsed metastatic disease, [44.8% had a metastasis stage of M0]. Only 22.2% of patients with locally advanced disease had received prior systemic therapy [vs 86.7% of patients with relapsed metastatic disease].16
The ORR was 50.0% [95% CI, 36.1%-63.9%] in the group with locally advanced disease and 35.2% [95% CI, 26.2%-45.2%] in the group with relapsed metastatic disease. For these same respective groups, the DCR was 64.8% and 52.4%. The median time to response was 2.6 months vs 1.6 months, respectively. The rates of CR were 16.7% vs 10.5%, [respectively], and the rates of PR were 33.3% vs 24.8%. The patients with metastatic disease achieved CR and disease control. [Overall], the different cohorts, though they had different risks, [had similar results]. Similarly to the EMPOWER-CSCC-1 study, [this study produced profound photographic evidence of drug efficacy, with locally advanced disease yielding to a CR and recurrent metastatic disease exhibiting a PR].16
REFERENCES
1. Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28(36):5321-5326. doi:10.1200/JCO.2010.27.9943
2. Harris L. Basal cell carcinoma: a pharmacist’s guide. US Pharm. 2019;44(8):29-35. August 19, 2019. Accessed September 21, 2022. https://bit.ly/3Em6tAd
3. Sekulic A. How to appropriately use Hedgehog in advanced basal cell carcinoma. Presented at: 2019 American Society of Clinical Oncology Annual Meeting; May 31-June 4, 2019; Chicago, IL. Accessed September 21, 2022. https://bit.ly/3Vb2mwQ
4. Mohan SV, Chang AL. Management of cutaneous and extracutaneous side effects of smoothened inhibitor therapy for advanced basal cell carcinoma. Clin Cancer Res. 2015;21(12):2677-2683. doi:10.1158/1078-0432.CCR-14-3180
5. Erivedge. Prescribing information. Genentech; 2020. Accessed September 21, 2022. https://bit.ly/3VejjXl
6. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/S1470-2045(21)00126-1
7. Libtayo. Prescribing information. Regeneron; 2021. Accessed September 21, 2022. https://bit.ly/3SJfWWw
8. Lacouture ME, Dréno B, Ascierto PA, et al. Characterization and management of Hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218-1229. doi:10.1634/theoncologist.2016-0186
9. NCCN. Clinical Practice Guidelines in Oncology. Squamous cell skin cancer, version 2.2022. Accessed September 21, 2022. https://bit.ly/3fRgo6E
10. Migden MR, Khushalani NI, Chang ALS, et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with locally advanced cutaneous squamous cell carcinoma (laCSCC). J Clin Oncol. 2019;37(suppl 15):6015. doi:10.1200/JCO.2019.37.15_suppl.6015
11. Rischin D, Khushalani NI, Schmults CD, et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): longer follow-up. J Clin Oncol. 2020;38(suppl 15):10018. doi:10.1200/JCO.2020.38.15_suppl.10018
12. Rischin D, Khushalani NI, Schmults CD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer. 2021;9(8):e002757. doi:10.1136/jitc-2021-002757
13. FDA approves cemiplimab-rwlc for metastatic or locally advanced cutaneous squamous cell carcinoma. FDA. September 28, 2018. Updated January 18, 2019. Accessed September 22, 2022. https://bit.ly/3yqYWfL
14. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341-351. doi:10.1056/NEJMoa1805131
15. Grob JJ, Gonzalez R, Basset-Seguin N, et al. KEYNOTE-629: phase 2 study of pembrolizumab for recurrent/metastatic or locally advanced unresectable cutaneous squamous cell carcinoma (cSCC). J Clin Oncol. 2019;37(suppl 15):TPS9598. doi:10.1200/JCO.2019.37.15_suppl.TPS9598
16. Hughes BGM, Munoz-Couselo E, Mortier L, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. 2021;32(10):1276-1285. doi:10.1016/j.annonc.2021.07.008

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More