Comparing Latest Evidence for Therapies in Newly Diagnosed Multiple Myeloma
During a Targeted Oncology case-based roundtable event, Adam Binder, MD, discussed trials supporting triplet and quadruplet regimens along with autologous stem cell transplant.
Adam Binder, MD
Medical Oncologist
Sidney Kimmel Medical College
Jefferson Health
Philadelphia, PA

Targeted OncologyTM: What are the recommended treatment regimens for transplant-eligible patients with newly diagnosed multiple myeloma (NDMM)?
BINDER: Based on the most recent NCCN [National Comprehensive Cancer Network] guidelines, the preferred regimen is still a triplet regimen with bortezomib [Velcade], lenalidomide [Revlimid], and dexamethasone [VRd]. The other recommended regimens are a quadruplet of daratumumab [Darzalex] plus VRd; [as well as] carfilzomib [Kyprolis], lenalidomide, and dexamethasone [KRd]; or ixazomib [Ninlaro], lenalidomide, and dexamethasone, although that’s a category 2B recommendation, so VRd in the NCCN guidelines is still up there as the preferred regimen.1
I will say, though, for [individuals] who use clinical pathway guidelines in their practices, the recommendations were changed to the quadruplet of daratumumab plus VRd [D-VRd] for all new diagnoses of myeloma, both in high-risk and standard-risk patients. I think different treatment algorithm pathways are adopting different things. There are different groups of [individuals] putting these together, so it seems like they’re both acceptable treatment options today.
What available data support the use of VRd followed by autologous stem cell transplant (ASCT) in transplant-eligible patients with NDMM?
One of the most seminal articles out there is the 2017 New England Journal of Medicine publication by Attal et al,2 which presented the IFM 2009 [NCT01191060] study data. This was a clinical trial looking at transplant-eligible patients with NDMM receiving 3 cycles of VRd with or without ASCT. All patients got 3 cycles of VRd and everyone got ASCT collected after that if they had any response. Cyclophosphamide [Cytoxan] was used for the collection. They were then randomized to receive ASCT with 200 mg/m2 of melphalan [Alkeran], followed by 2 more cycles of consolidation, or 8 cycles of VRd. Everybody then received lenalidomide maintenance therapy for 12 months starting at 10 mg/d for 3 months, and if they were tolerating it well, [then] it was increased to 15 mg/d for the subsequent 9 months.
The initial publication in 2017 had a 44-month follow-up and it showed that [patients given] up-front ASCT compared with no ASCT had a median progression-free survival [PFS] improvement of about 14 months. Those who got the ASCT had a median PFS of 50 months and those who didn’t had a median PFS of 36 months.2 For the follow-up data of almost 90 months, the numbers changed slightly. There was an absolute benefit of about 1 year in terms of PFS in the up-front transplant group. Everybody was interested in what the long-term follow-up would be because even though the PFS was better with the initial transplant…in the nontransplant arm about 77% of patients ultimately received an ASCT at their first relapse.
The big question was [whether] this study not only looked at whether transplant up front was better, but also whether transplant at first relapse normalized out that risk. Looking at the PFS was important as well. When you have a 90-month follow-up and you’re 8 years out, the overall survival [OS] is pretty much the same in both groups, with a 60.2% survival in the VRd alone group and a 62.2% survival in the up-front transplant group.3 Some subgroup analyses show the standard-risk patients did a bit better, but we tend to see that in a lot of our myeloma trials.
Similarly, they looked at response rates and PFS by minimal residual disease [MRD] status alone. Patients who received VRd without ASCT and who were MRD negative at the end of those initial 8 cycles did much better than patients who received up-front ASCT and were MRD positive….In addition, there was a higher percentage of patients who were MRD negative after the transplant than those who received VRd alone. Overall, more patients are getting this PFS benefit. What’s important to note is that for the patients who received VRd alone and then got ASCT at first relapse, their absolute PFS benefit was about 11 months compared with the patients who got transplant up front.3
When you look at the second PFS, the 2 groups kind of evened out. It seems like a salvage transplant in this patient population was just as good as an up-front transplant. I think this study answered 2 questions: One, that transplant is important, but 2, that maybe the timing of transplant doesn’t matter that much. Whether you get it up front or at first relapse, the benefit is there regardless. So, [in this patient case], it’s maybe less of an issue. For someone in their 70s where they may not be transplant eligible in 6 or 7 years, one may want to think about giving them a transplant earlier rather than later.
What available data support the use of KRd followed by ASCT in transplant-eligible patients with NDMM?
The FORTE trial [NCT02203643] looked at carfilzomib in combination with cyclophosphamide and dexamethasone [KCd], or [KRd]. There were 3 arms in this study. The first 2 arms were comparing KCd vs KRd, followed by a single ASCT. The third arm looked at 12 cycles of KRd to try to answer the question of whether 12 cycles of care could do just as good as care plus ASCT. This study used the old carfilzomib dosing at 36 mg/m2 on days 1, 2, 8, 9, 15, and 16. Cyclophosphamide was given weekly on days 1, 8, and 15 at 300 mg/m2. Dexamethasone was given at 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23. So, essentially 40 mg/wk. In the KRd arm, lenalidomide was 25 mg on days 1 to 21, and these were all 28-day cycles across the board.
All patients received 4 cycles of their allotted treatment and then received stem cell mobilization. The patients in the first arm got 4 cycles of KCd followed by transplant and then 4 more cycles of KCd consolidation. Then one of the KRd arms had the same thing, 4 cycles of KRd followed by transplant and 4 cycles of KRd consolidation. The other arm got 12 cycles straight of KRd. The patients had a second randomization to either receive lenalidomide [R] maintenance 10 mg on days 1 to 21 with 7 days off until progression or intolerance, or they were randomized to receive carfilzomib every other week on days 1 and 2 and days 15 and 16 for up to 2 years plus lenalidomide [KR] on days 1 to 21 until progression or intolerance.4
After a median follow-up of 36 months, the median PFS for KRd followed by transplant followed by KRd consolidation was not reached, the median PFS for 12 cycles of KRd was 57 months, and the median PFS for KCd plus ASCT followed by KCd consolidation was 53 months. KRd followed by ASCT had a superior PFS compared with KCd plus ASCT [HR, 0.53; 95% CI, 0.37-0.77; P < .001] or 12 cycles of KRd [HR, 0.64; 95% CI, 0.44-0.94; P = .023]. It seems like KRd is better than KCd hands down.4
There are other studies showing that immunomodulatory drugs [IMiDs] are better than alkylating agents and up-front therapy….When looking at [whether] we can get rid of ASCT and just do 12 cycles of KRd, it seems like, from a PFS perspective at least, ASCT is still needed even with the use of carfilzomib to improve the depth of response and duration of response and improve PFS up front. This affirmed that regardless of what the initial line of therapy is, there seems to be an added benefit to PFS when ASCT is added to a triplet.
The subgroup analyses do seem to be a hint that every group is favored with KRd plus ASCT, but none of them hit statistical significance; overall though, there is a clear PFS benefit. There was a 3-year OS report at initial publication and it looked like KRd plus ASCT and 12 cycles of KRd had similar survivals at about 90% and KCd plus ASCT had a survival of 83%.4 So, [it is] a little too early to tell how those [results will end up], but there’s sort of a signal that using a KRd-based regimen up front seems to have better trending toward some improved survival. The second randomization, which was the maintenance portion of the study, looked at 356 patients.
The premaintenance response had balance across both arms. There were good CR [complete response] rates in the KR and R arms—about 62% and 59%, respectively. The stringent [CR] was about the same, and MRD negativity was pretty much equal in both arms. So, in terms of depth of response, one is starting from the same place with these 2 groups. KR, maybe not surprisingly, has a better PFS at 3 years compared with R [HR, 0.63; P = .026].4 This just reaffirms [individuals’] feeling that for patients who are at a higher risk for relapse, maybe giving that doublet of a proteosome inhibitor and an IMiD may help prevent relapse from occurring soon.
What available data support the use of the quadruplet daratumumab, lenalidomide, bortezomib, and dexamethasone (D-VRd) followed by ASCT in transplant-eligible patients with NDMM?
There is a more recent study that’s been published and is kind of the crux of a lot of the conversation about a quadruplet regimen with the fourth drug being daratumumab. The randomized phase 2 GRIFFIN trial [NCT02874742] had D-VRd vs VRd in transplant-eligible patients with NDMM. For the key eligibility criteria, patients were [aged] 18 to 70 [years]. They had an ECOG performance status of 0 to 2, and creatinine clearance greater than 30 mL/min, just to allow for adequate lenalidomide dosing.
They all had to be deemed transplant eligible and interested in up-front ASCT. So, there was a 1:1 randomization between the 2 arms. The daratumumab was given IV [intravenously] in this study at 16 mg/kg/wk on days 1, 8, and 15; lenalidomide was given at 25 mg on days 1 to 14; bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, and 11; and dexamethasone was given at a total dose of 40 mg/wk, split as 20 mg on days 1, 2, 8, 9, 15, [and] 16 of a 21-day cycle.
The VRd arm essentially got the same thing without the daratumumab. All patients received 4 cycles of their respective regimens and then received stem cell mobilization with GCSF [granulocyte colony-stimulating factor] plus or minus plerixafor [Mozobil] and went to transplant. They all then received consolidation for 2 cycles, so cycles 5 and 6 were consolidation, and then went on to the maintenance phase for the next 26 cycles.5
Note that there was no second randomization at the maintenance portion of this study. Everybody who was on D-VRd in the beginning got D-R as maintenance and everyone who was on the VRD arm got R maintenance. So, they weren’t trying to answer the second question about maintenance therapy. I think it’s also important to note that there was a lower percentage of patients in the VRd arm who went on to transplant. So, in the D-VRd arm, 90% of patients went on to transplant, whereas only 76% went on to transplant in the VRD arm. This was due to early discontinuations because of relapse or response. About 15% of patients met the criteria for high-risk [MM], which is standard in a lot of the trials. The primary end point was stringent CR by the end of consolidation and secondary end points were NGS [next-generation sequencing]–based MRD status, at a sensitivity of 10–5 by IMWG [International Myeloma Working Group] criteria, CR, overall response rate [ORR], and percentage of patients who had responses greater than VGPR [very good partial response].5
The ORR results were impressive in terms of just how much of a better, deeper response the quadruplet regimen had compared with the triplet regimen at all phases of assessment from the end of induction, transplant, consolidation, [and] 1-year and 2-year maintenance. Both arms had deepening of responses all the way up to about 1 year post maintenance. It seems like once [patients] get to 1-year maintenance, their responses stabilized out and there wasn’t too much of a difference between 1 and 2 years of maintenance therapy. But we see deepening responses across the board. D-VRd through all phases had much deeper responses, more stringent CR, as high as 63% at the end of 1 year of maintenance, and a greater than 80% CR rate after 1 year of maintenance, compared with a 60% CR rate in the VRd alone arm. The median follow-up at 24 months of maintenance therapy cutoff was 38.6 months.6
The MRD negativity rates by NGS were assessed by adaptive clonoSEQ technologies, even though clonoSEQ measures to 10–6. By IMWG criteria, 10–5 is MRD negativity. There was a deepening of response particularly when looking at MRD negativity, even after 2 years of maintenance. A higher percentage of patients were continuously becoming MRD negative, showing the added benefit of ongoing maintenance therapy. Between 1 and 2 years, an extra 15% of patients received an MRD-negative status, based on a 10–6 threshold.6
How do the MRD and PFS results impact the decision to use this treatment?
The subgroup analysis of patients who received MRD negativity after 2 years of maintenance therapy seems to favor the quadruplet regimen across the board, although there is the question of high-risk cytogenetics and whether they benefit the same way as the standard-risk patients do. It’s clear that the standard-risk patients get a benefit of the quadruplet over triplet regimen. It’s not as clear in the study alone whether the high-risk patients do, although there has been some meta-analysis of quadruplet regimens, including daratumumab, that have shown a benefit in larger patient populations. The number of patients in the high-risk groups was relatively small in this study, so it’s hard to form any real statistical conclusions.
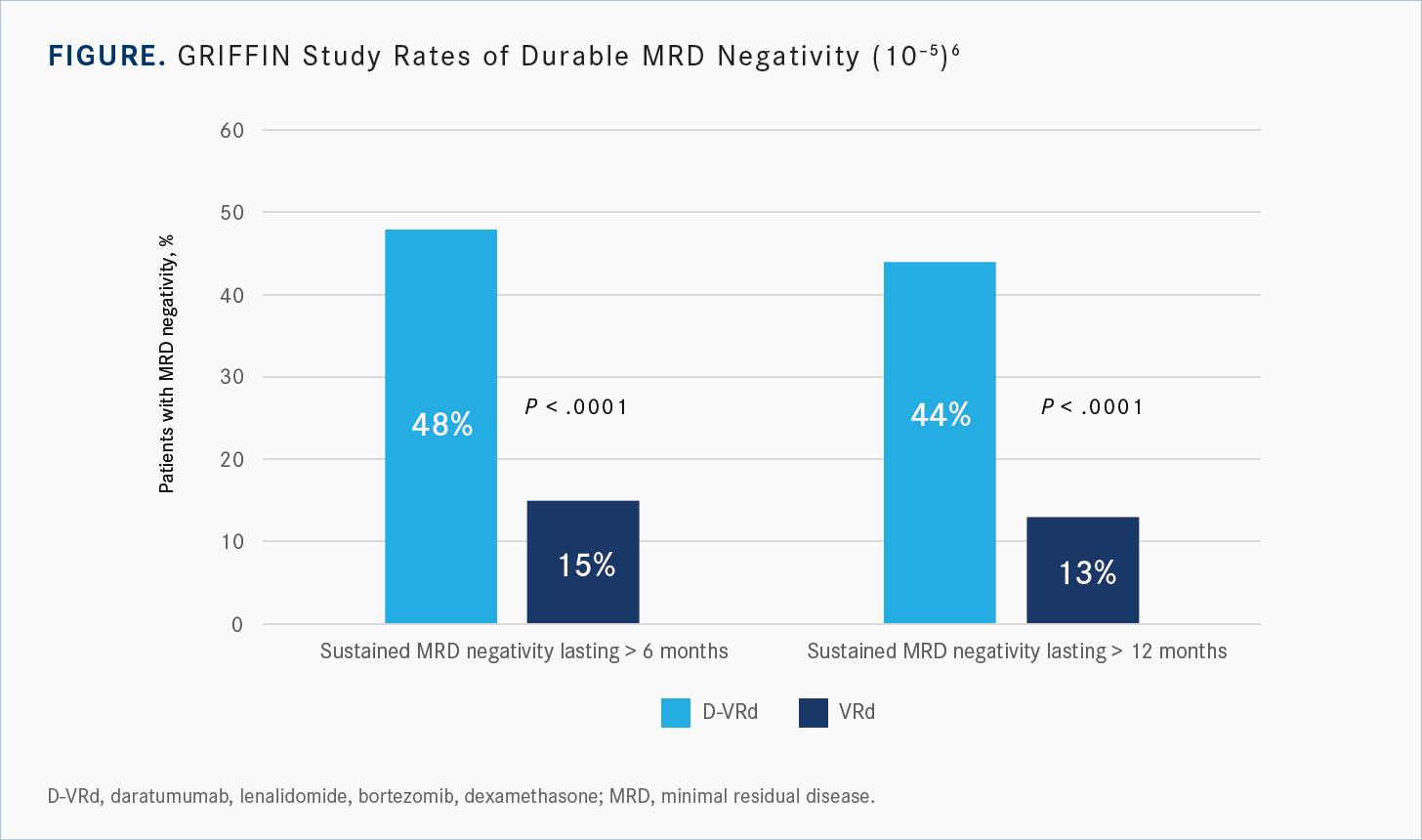
The MRD-negativity rates were quite durable on the quadruplet regimen, and patients sustained that negativity over time, so there was this durable response, both at 6 months and 12 months [Figure6]. Patients who got the triplet regimen had a higher percentage of MRD negativity post consolidation at about 20% to 24%, but only 15% of them had sustained MRD negativity at 6 months and 13% had sustained MRD negativity at 12 months.6 So, we’re seeing this kind of loss of response over time with the triplet regimen that you’re not necessarily seeing quite as much of with the quadruplet regimen. A durable MRD is important, so I think it is important not just to look at MRD at one point in these studies, but how well the patients sustain their MRD-negative status over the follow-up period.
The PFS in the intention-to-treat population had both arms do quite well. At 36 and 48 months, neither group had reached a median PFS. The numbers looked a lot better than the IFM 2009 study. The median follow-up was only 38.6 months at the time of the last reporting at ASH [American Society of Hematology Annual Meeting] 2021 and the median PFS wasn’t reached yet in either group. The VRd arms are still doing well, but there does seem to be a trend toward an improvement in PFS for the quadruplet therapy vs the triplet therapy. The separation [of results] starts beyond 1 year of maintenance, again reflecting that durable MRD negativity and durable responses that we saw with the doublet maintenance therapy as opposed to single maintenance therapy. Probably it also reflects the increased depth of response with the quadruplet over the triplet regimens.
Are there any safety signals to consider?
When thinking about treatment, efficacy is important, but toxicity is just as important. Cytopenia is more common when adding daratumumab to any combination. There’s about a doubling of the percentage of grade 3 or 4 neutropenia and thrombocytopenia in the patients receiving quadruplet regimens. Also, for leukopenia, there’s a higher percentage and about 9.1% of patients had grade 3 or 4 anemia on the quadruplet compared [with] 5.9% in the triplet. It was about the same for lymphopenia.
There is also a higher incidence of infections in the quadruplet arm. We often think about daratumumab increasing the risk of upper respiratory tract infections [URTIs] or pneumonias. But when you look at it a little more deeply, even though it was about a 30% higher incidence of infections at any grade, most of them were just grade 1 or 2 URTIs. Severe grade 3 or 4 infections that resulted in hospitalizations were around 20%.5,6 There’s a lot more viral and maybe bacterial sinusitis that you must deal with, but not necessarily resulting in increased hospitalizations or severe infections.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 4.2021. Accessed January 29, 2021.
2. Attal M, Lauwers-Cances V, Hulin C, et al; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
3. Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 Trial. Blood. 2020;136(suppl 1):39. doi:10.1182/blood-2020-134538
4. Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(suppl 15):8002.doi:10.1200/JCO.2019.37.15_suppl.8002
5. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
6. Laubach JP, Kaufman JL, Sborov DW, et al. Daratumumab (dara) plus lenalidomide, bortezomib, and dexamethasone (VRD) in patients (pts) with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of GRIFFIN after 24 months of maintenance. Program and abstracts of the 2021 American Society of Hematology Annual Meeting; December 11-14, 2021; Atlanta, GA. Abstract 79.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More