Westin Discusses the Evidence for Using New Therapies in R/R DLBCL
During a Targeted Oncology case-based roundtable event, Jason Westin, MD, discussed recent clinical trial data for patients with relapsed/refractory diffuse B-cell lymphoma.
Jason Westin, MD
Director, Lymphoma Clinical Research
Section Chief, Aggressive Lymphoma
Department of Lymphoma/Myeloma
The University of Texas MD Anderson Cancer Center
Houston, TX

Targeted OncologyTM: What therapies do the National Comprehensive Cancer Network (NCCN) guidelines recommend for patients with DLBCL who have relapsed within 12 months or who have primary-refractory disease?
WESTIN: [The first option to consider is] chimeric antigen receptor [CAR] T-cell therapy.1 The FDA has approved axicabtagene ciloleucel [axi-cel; Yescarta],2 and the FDA is likely to review the data for lisocabtagene maraleucel [liso-cel; Breyanzi] soon, which may [provide us with] more than 1 option for CAR T-cell therapy [in this setting]. Right now, [we are acting on] the data from the ZUMA-7 trial [NCT03391466], which showed the superiority of axi-cel over high-dose chemotherapy followed by autologous stem cell transplant [ASCT] in patients who had relapsed within 12 months or who had primary-refractory disease.
What data support the use of CAR T-cell therapy in patients with relapsed or refractory DLBCL?
Data from the ZUMA-7 trial of axi-cel and the BELINDA [NCT03570892] trial of tisagenlecleucel [tisa-cel; Kymriah] were published in the same issue of the New England Journal of Medicine.3,4 An interim analysis of the data from the TRANSFORM trial [NCT03575351] of liso-cel also has shown [CAR T’s potential in this patient population].5
All 3 of these studies were randomized trials of CAR T-cell therapy vs high-dose chemotherapy and [ASCT] for those patients who responded [to the chemotherapy]. All the studies enrolled patients who were less than 12 months from completion of first-line therapy.3-5 All the patients [in these trials] were potentially going to receive a stem cell transplant. The TRANSFORM trial had an upper age limit [for transplant] of 75 years.5 The other trials did not have an upper age limit but [rather left the decision of transplant candidacy to the discretion of the treating physician].3,4
[With regard to the use of] bridging therapy [in an attempt to control the] disease, the ZUMA-7 trial allowed for glucocorticoid therapy only, with no bridging chemotherapy.3 But the BELINDA trial and the TRANSFORM trial both allowed bridging chemotherapy.4,5 In the BELINDA trial, 83% of patients received at least 1 cycle of chemotherapy as bridging therapy.4 In the TRANSFORM trial, [63%] of the patients received bridging therapy.5 So a lot of bridging therapy was used [in these 2 trials], but in the ZUMA-7 trial, [36%] of the patients received glucocorticoids only, and this difference [in bridging therapy] was largely due to the speed of delivery of the CAR T cells.3
In the ZUMA-7 trial, the CAR T cells were turned around in 3 or 4 weeks, from apheresis to infusion. For the BELINDA trial, it was [closer to a] 50-day turnaround.4 Bridging therapy is often used for CAR T cells outside clinical trials, and I do not think that will be a major difference in using the T cells going forward.
In the ZUMA-7 trial, [74%] of the patients were refractory to initial chemotherapy.3 The TRANSFORM trial was similar, with 73% of patients refractory to first-line therapy.5 And in the BELINDA trial, [66%] of the patients were refractory.4 Many of these patients had never responded to chemotherapy [and were now] being [randomly assigned] to high-dose chemotherapy vs CAR T-cell therapy. The reason [these data are worth discussing is] the HRs for event-free survival, all favoring CAR T-cell therapy.
For the ZUMA-7 trial, the HR was 0.4 [95% CI, 0.31- 0.51; P < .001].3 For the TRANSFORM trial, the HR was [0.349; 95% CI, 0.229-0.530; P < .0001].5 The TRANSFORM data reflect the results of a preplanned interim analysis at only 6 months of follow-up, so those data are not as mature as we would like. However, the ZUMA-7 data reflect the results of 24 months of follow-up, and this trial produced a very impressive 24-month progression-free survival [PFS] of 46% in the experimental arm vs 27% in the control arm.3
These data led the FDA to deem that axi-cel was appropriate for second-line treatment of patients who relapsed within 12 months.2 [Please note that] if you have a patient who has relapsed beyond 12 months from first-line therapy, that patient [is not included in the approved population]. But if you get a patient who does not respond to R-CHOP or who relapses 9, 10, or 11 months afterward, they now could be a candidate for CAR T-cell therapy instead of chemotherapy and ASCT.
What do the NCCN guidelines recommend for patients who are not candidates for transplant or CAR T-cell therapy?
The recommended regimens include the combination of GemOx [gemcitabine (Infugem) and oxaliplatin (Eloxatin)] with or without rituximab.1 We have used that before with some effectiveness, but it is still a platinum-based chemotherapy. Another option is polatuzumab vedotin-piiq [Polivy] with or without bendamustine [Bendeka] and with or without rituximab. Polatuzumab vedotin is an antibody-drug conjugate targeting CD79B and is approved to treat [adult patients with] large cell lymphoma who have progressed after 2 or more prior lines of therapy.6 The combination of tafasitamab-cxix [Monjuvi], which targets CD19, plus lenalidomide [Revlimid] is a third option. This combination is approved by the FDA to treat adults with relapsed or refractory DLBCL who are not eligible for transplant.7
What do the NCCN guidelines recommend for patients who do not respond to second-line therapy?
For these patients, all 3 of the FDA-approved CAR T-cell therapies are on the list. Other options are loncastuximab tesirine-lpyl [Zynlonta], a CD19-targeting antibody-drug conjugate, and selinexor [Xpovio], the oral XPO1 inhibitor.1 It is important to note that it is unclear whether any [CD19-targeting] therapy is likely to have an impact on subsequent CAR T-cell therapy, [which has the same target].
In my opinion, [CD19-targeting therapies] are not likely to have [an impact on subsequent CAR T-cell therapy]. I think that the antibodies just do not have the selection pressure [necessary] to impact the expression of CD19 in a way that would cause the CAR T cells not to work. That is [based on] anecdote. Some physicians might prefer to target something other than CD19 if they intend to use CAR T-cell therapy, [but I think] it is not likely to matter much.
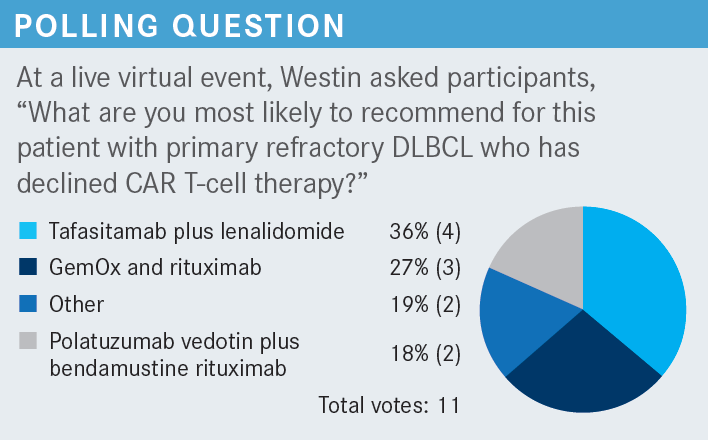
What data support the use of tafasitamab plus lenalidomide in patients with relapsed or refractory DLBCL?
The L-MIND [NCT02399085] phase 2 clinical trial [investigated the use of this combination] in patients who had relapsed or refractory DLBCL and who had received 1 to 3 prior regimens. Initially, patients with primary-refractory disease were enrolled in the study.
After the first cohort was enrolled, there was a change in eligibility such that patients with primary-refractory disease were excluded. A few patients in this study might have been primary refractory, but most patients on this data set were not primary refractory and were not intended for transplant.8,9
The first 3 cycles of treatment consisted of weekly tafasitamab infusions. Next, patients switched to infusions every 2 weeks, on day 1 and day 15, [through] cycle 12. During those first 12 cycles, patients also received lenalidomide, 25 mg daily, on days 1 through 21 of a 28-day cycle, which is a standard [dosage] pattern that has been used for other diseases.
If the patients had a clinical benefit, defined as [CR] or partial response [PR], they could continue on maintenance tafasitamab beyond cycle 12 until disease progression. [The maintenance regimen consisted of tafasitamab administered every other week.8,9] Some might point out that the dose of lenalidomide was a relatively high dose for what could be considered an older or frail patient population. A fair amount of dose modifications is done in clinical practice [to manage] cytopenias and other toxicities. That is something that I often do when I use this combination.
Can you describe the makeup and results of the study?
There were 81 patients on the study. The median age was 72 years, and the [oldest] patient was aged 86 years.8,10 Patients with primary-refractory disease comprised only a [small fraction] of the population, so the L-MIND trial was conducted mostly in [patients with] relapsed disease. This is in contrast with the population in the ZUMA-7 trial, of whom 75% had [primary-refractory disease].3 Finally, only 11% of the patients in the L-MIND trial had received a prior transplant. It is important to note that this was not a heavily pretreated group of patients.8,10
The results showed that 40% of the patients had a CR and 17.5% had a PR, so more than half of the patients had a response. The median duration of response [DOR] was 43.9 months. [This result] was largely driven by the patients with CRs, which is good because those patients who benefit tend to get a durable benefit.
By 42 months [of follow-up], among patients who had a CR, approximately 80% still had not progressed [median DOR, not reached (NR)]. That is incredible. Patients who had less than a CR did not do quite as well. The median DOR for patients with a PR was 5.6 months [95% CI, 2.2-NR],10 but for those patients who achieve a CR, a durable response is possible with this targeted therapy combination.
Median PFS was 11.6 months [95% CI, 6.3-45.7], and the median [OS] was 33.5 months [95% CI, 18.3-NR],10 which is quite impressive for a population who was not intended for transplant and who historically has not done very well with standard chemotherapy. According to the old NCCN guidelines, [patients who were not] intended for transplant or who relapsed after transplant [had no options other than] supportive or palliative care; [there was no option that offered] a 33.5-month median OS.
[The efficacy was examined with respect to] how many [prior] treatments each patient had received [1 vs 2 or more], [and the investigators found,] not surprisingly, that those patients who were less heavily pretreated tended to [respond] better. The overall response rate in the less heavily treated population was 67.5% [95% CI, 50.9%-81.4%] and in the more heavily treated population was 47.5% [95% CI, 31.5%-63.9%; Table11].
What adverse events (AEs) should be highlighted from this treatment?
The AEs observed in this study were [similar to] those observed with other lenalidomide-based combinations. [Among these] were cytopenias. Neutropenia of grade 3 or grade 4 affected [27% and 21% of the patients, respectively]. Anemia of grade 1 or grade 2 collectively affected 27% of patients, and thrombocytopenia of grade 1 or grade 2 collectively affected 14% of patients. Febrile neutropenia [was] relatively rare, affecting only 12% of the patients in this 80-patient study. Although there was significant neutropenia, it did not [often] result in a fever or an admission to the hospital.8,12 Overall, the AEs were things that [we see regularly].
Among the nonhematologic AEs, [not many were] grade 3 or grade 4. Some patients [9%] did have a grade 3 rash. Grade 1 and grade 2 rashes [collectively affected 27% of patients, and such rashes] may be managed with a dose hold of lenalidomide [or] potentially with some topical or systemic steroids [plus] lenalidomide dose reduction. But the fatigue, diarrhea, cough, [and so on] are things that we [frequently see because of chemotherapy-based regimens], and we can manage them with supportive care.8,12
Serious AEs affected 51% of the patients, though only 19% experienced AEs that were thought to be therapy related; that is quite impressive to me. Discontinuations of the combination due to toxicities affected only 12% of the patients; that [suggests that] these toxicities were generally manageable. Finally, of 30 deaths, 4 [13%] were attributed to therapy.8,11,12 The AEs were analyzed with respect to the timing of the therapy. Recall that the tafasitamab plus lenalidomide combination was given for the first 12 cycles, followed by tafasitamab monotherapy.
The AEs occurred much more frequently during the combination [phase] than during the monotherapy [phase]. This implies that the combination of the tafasitamab plus lenalidomide was much more toxic than [tafasitamab] by itself.9 I think much of the toxicity was driven by the lenalidomide. That is why I [suggest that you consider] dose reducing this treatment if your patient is having trouble.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 5.2022. Accessed September 20, 2022. https://bit.ly/3V3BzCE
2. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma. FDA. April 1, 2022. Accessed September 20, 2022. https://bit.ly/3CxZP8L
3. Locke FL, Miklos DB, Jacobson CA, et al; all ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. doi:10.1056/NEJMoa2116133
4. Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629- 639. doi:10.1056/NEJMoa2116596
5. Kamdar M, Solomon SR, Arnason J, et al; TRANSFORM Investigators. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294-2308. doi:10.1016/S0140-6736(22)00662-6
6. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed September 20, 2022. https://bit.ly/2XEazLP
7. FDA approves Monjuvi (tafasitamab-cxix) in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). News release. MorphoSys AG. July 31, 2020. Accessed September 20, 2022. https://bwnews.pr/3SXkx7a
8. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
9. Salles G, Duell J, González Barca E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Hematol Oncol. 2019;37(52):173-174. doi:10.1002/hon.130_2629
10. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958
11. Düll J, Maddocks KJ, Gonzalez-Barca E, et al. Long-term analyses from L-MIND, a phase II study of tafasitamab (MOR208) combined with lenalidomide (LEN) in patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). J Clin Oncol. 2021;39(suppl 15):7513. doi:10.1200/JCO.2021.39.15_suppl.7513
12. Monjuvi. Prescribing information. Morphosys US; 2021. Accessed September 20, 2022. https://bit.ly/3ygpLmI
