Roundtable Discussion: Stinchcombe Reviews Second-Line Therapy for ES-SCLC
During a Targeted Oncology case-based roundtable event Thomas E. Stinchcombe, MD, discussed the case of a 53-year-old patient with extensive-stage small cell lung cancer.
Thomas E. Stinchcombe, MD, discussed the case of a 53-year-old patient with extensive-stage small cell lung cancer during an cased-based roundtable event.
MOHAMED: Platinum-sensitive is if the recurrence occurs more than 6 months post treatment. Less than 3 months, or within 3 months, that’s refractory. And 3 months to 6 months is a gray area where you can play around, but I would definitely consider 3 months to 6 months resistant, honestly, but 6 months definitely is sensitive.
Thomas E. Stinchcombe, MD


PATI: Six months for platinum sensitive.
RABARA: I personally would use 6 months also.
STINCHCOMBE: How many of your patients make that 6-month cutoff?
RABARA: Personally, I think most of them do. I’d say probably greater than 50%, in my experience.
STINCHCOMBE: Is that consistent with other people’s experience?
NATHWANI: We have half of patients [who are platinum sensitive].
OSEI-BOATENG: That sounds about right.
MOHAMED: Yes, it’s very clear, even from the frontline clinical trial, even with the immune therapies, that PFS [progression-free survival] is around 5 months.
STINCHCOMBE: Yes, right. I think that it also depends on when you scan. I tend to personally scan for the first 2 cycles, and the fourth cycle, then I space them out a little bit. So that might bias me; should I do a scan at 3 months? Or earlier if symptoms dictate? Now this patient has had a durable benefit of 7 months.
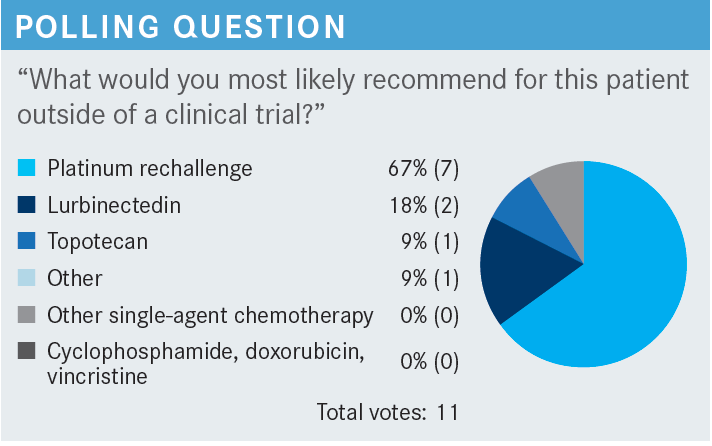
MOHAMED: A question on this patient: Did he continue maintenance immunotherapy, and then he progressed after 7 months?
STINCHCOMBE: For this discussion, yes, he was on maintenance therapy. For the participants who chose platinum rechallenge, perhaps we can ask one of you to explain your rationale.
PATI: [I would choose platinum rechallenge] since it was more than 6 months, so hopefully the patient will still be sensitive to platinum.
STINCHCOMBE: Then, for someone who chose lurbinectedin [Zepzelca], why don’t you explain why you chose it.
KATRAGADDA: I chose lurbinectedin just because it’s more tolerable, easy to administer, and has reasonably good response rates in that setting, about 40% [From the Data].1 I had good experiences with it, so that’s 1 other thing. It’s also much better than topotecan.
STINCHCOMBE: [Dr Hanna], could you explain why you chose topotecan?
HANNA: I chose topotecan because I feel this patient is platinum resistant. After 7 months, this patient had high-volume, multiple-organ disease. I just felt this is not a typical 6-month period, so to speak, so I felt maybe he’ll get a better response if I use a different agent, so I chose topotecan. At this point, in the second line, there is nothing good for those patients, but I had a good experience with topotecan. I’ve used lurbinectedin before. Maybe I’m better at managing adverse events [AEs] for topotecan than lurbinectedin.
KUZMA: We have clinical trials for all stages of relapsed SCLC, but I would choose platinum rechallenge otherwise.
MOHAMED: When you have platinum rechallenge, do you keep the immune therapy, or do you change it, or do you discontinue it?
STINCHCOMBE: I would do the platinum/etoposide/immunotherapy, then immunotherapy maintenance. Then if they make it for longer than 6 months, my cutoff—I realize that that’s debatable—I will do platinum/etoposide alone, because they’ve progressed on immunotherapy, and I think there’s no evidence of retreatment with immunotherapy at this point or continuing treatment beyond progression in this disease. I have not done that. What do others do?
RABARA: I probably would skip the immunotherapy as well and just do the platinum and etoposide.
STINCHCOMBE: It’s a good question, though I don’t think anyone knows the appropriate answer. Dr Thomas, would you re-treat with immunotherapy?
THOMAS: No, I would not. I agree with exactly what you said you would do: I’d drop the immunotherapy, and then I would rechallenge with etoposide and carboplatin.
STINCHCOMBE: I think the National Comprehensive Cancer Network [NCCN] guidelines reflect the sentiment of the group, if you look at it. For relapse before 6 months, the preferred therapies are topotecan, lurbinectedin, or a clinical trial. For relapse beyond 6 months, the original regimen is the standard at this point.2
The NCCN guidelines give you lots of flexibility for other options that people may use at this point. I’ve used CAV [cyclophosphamide, doxorubicin (Adriamycin), and vincristine] in the third-line setting; in a healthy patient, that’s an active combination. I think the challenge with this disease is that topotecan was approved in 1996 and lurbinectedin was approved in June 2020.3,4
So outside of immunotherapy, we’ve not had a lot of new drugs approved, particularly—until lurbinectedin was approved in June of 2020, we didn’t have any new drugs in the second-line setting with FDA approval. Remember that doxorubicin was very promising for a period of time; that didn’t work out, so this is a challenging area of drug development.

MADADI: I usually see around 2 to 3 months when they initially respond, and after 2 or 3 cycles you definitely start seeing progression.
STINCHCOMBE: So it has a reasonable response rate, but not the durability; seems to be less time to relapse than the first time.
OSEI-BOATENG: Yes, I was going to say the same thing. Usually, by 4 cycles, they’ve started to progress. That’s the typical longest that I've seen.
STINCHCOMBE: I would say, also, I think the myelosuppression’s slightly worse the second time, in my experience, but that might just be my patient population.
STINCHCOMBE: Is there any first-line toxicity that would prevent retreatment, or any event that would make you think that this patient is not a candidate for double-agent therapy, and maybe needs something else?
RABARA: If the patient tolerated platinum very poorly initially, I may be hesitant on rechallenging them with the same regimen. I may just go to lurbinectedin in that case.
STINCHCOMBE: Now what do you all do with your performance status 2 patients? Does the borderline performance status affect your question of double agent vs single agent?
MOHAMED: Yes, patients on the later stage, after the first line, they don’t do well. Their bone marrow was already suppressed with the first-line therapy. So if I have a patient with a performance status of 2, initially I would treat that patient because I know that I can control the disease and get them to feel better with the front line. But a [patient with a] performance status of 2 on the second- and third-line setting is unlikely to do well compared to the first line.
STINCHCOMBE: If you have you used lurbinectedin, what types of patients have you used it for? How do you consider it for patients who are platinum sensitive?
MOHAMED: I have used it in many of my patients. I did not see an impressive PFS; the response rate was acceptable the first few cycles but gets lost after that. It’s an option that I’m going to continue to use, because I have not used topotecan since I finished my fellowship, and I’m not planning to use it. So definitely, it’s an option for me.
KATRAGADDA: I think it’s my preferred second-line treatment. Even if the response rates and toxicities are similar, it’s just not quite as unpopular as topotecan.
PATI: I agree with my previous colleagues that it’s much easier than topotecan.
STINCHCOMBE: Has the toxicity profile matched what was reported in the trial?
KATRAGADDA: I think topotecan has much more myelotoxicity and the chance of neutropenic infections [is greater] compared with lurbinectedin.
STINCHCOMBE: Do you use a granulocyte colony-stimulating factor prophylaxis when you use the lurbinectedin?
KATRAGADDA: I have not. I have used it only in patients who already had beaten-up bone marrow and a low white blood cell count to start with. I have used it on 1 patient, probably—that’s it. Otherwise, they do pretty well without it.
RABARA: I have only had limited experience with it so far, so I haven’t had an extensive experience like others have.
OSEI-BOATENG: I’ve used it in a few patients. I like it better in the second line because it’s much better tolerated compared with topotecan. I’ve only had to use growth factors in 1 patient. I never start out with growth factor support but I had to use it in a patient who ended up with neutropenic fever. So overall, I like it, and I think it’s my go-to second-line drug.
STINCHCOMBE: Do people use the platinum-free interval to triage the lurbinectedin as a choice? What are people’s thoughts on that?
OSEI-BOATENG: Yes, I typically use the 6 months. My go-to is to rechallenge; I think it’s just a matter of trying to use all the options you have. Eventually, it buys you another 3 or 4 months before you get to second line, if their performance status is still decent, and they tolerate it well.
KUZMA: We participated in the ATLANTIS trial [NCT02566993] with 2 patients, and neither did particularly well. In my experience, the few times I have used it now that it’s FDA approved, is that usually at the time of first assessment, radiographic assessment, they have progressed. However, it is a lot easier to give than 5-day-a-week topotecan that usually requires growth factor support.

MORSE: Irinotecan does have some CNS [central nervous system] penetration, and some of my patients with extrapulmonary SCLC have had brain metastases. So I think that’s useful, but I don’t know enough of the data on lurbinectedin. Are you confident of its CNS effects?
STINCHCOMBE: I don’t think it’s been formally assessed at this point because the phase 2 trial excluded those patients.1 I do think it’s interesting that the brain metastasis can be a problem in this patient population with multiple sites of progression. Sometimes, I’ve used temozolomide [Temodar], which is not a great drug, but was developed for CNS penetration. So I guess the question is what is the third-line therapy?
MOHAMED: Dr Morse brings a good point: You could always use irinotecan as a single agent in the third-line setting. Before the approval of lurbinectedin, and for patients who have platinum-sensitive disease, sometimes I used the combination of cisplatin at reduced doses and irinotecan, and I actually had impressive responses with this regimen.
I have not used it recently, because of lurbinectedin’s approval, but it was a good choice for me with a reduced dose on day 1 and 8 every 3 weeks, and I have had great responses with it. So I appreciate that Dr Morse brought this back to life, because we have used it. Or you can use it as single-agent irinotecan.
STINCHCOMBE: I’ve used it as well, and some people do very well, it seemed, after relapsing. Sometimes you need something that has some activity. But it’s not a tolerable schedule, no.
I think that reflects NTN [non–triple negative]: It gives you a wide flexibility because after you’ve gone through the FDA approvals, there’s not a lot of options that are clear cut better than the others.

MORSE: I like the idea of extending the interval between the platinum treatments and doing a mechanism of action switch first.
THOMAS: I just think the drugs are so ineffective in general in these second- and third-line patients. I don’t know. I guess you’d have to study it and figure out which one is more effective. I couldn’t say that I would prefer one or the other at this point, based on the lack of a clinical trial.
MOHAMED: Just to be on the opposite side of Dr Morse, I will choose platinum rechallenge followed by mechanism of action switch. I think if it’s more than 6 months, I think platinum challenge is a reasonable option to start with, and then you leave the other option for later.
KUZMA: In my experience, the patients who make it to the third line are those who got the second-line platinum rechallenge because they had a long interval. Usually, it’s almost always, in my experience, the ones that start with platinum rechallenge before trying a mechanism of action switch. I think we tried extending the platinum-free interval, in ovarian cancer, and that didn’t work out particularly well.
OSEI-BOATENG: In my experience, by the time patients get to third line, their performance status is also not that great for platinum rechallenge. I find that those who’ve had 6 months, they’re still in pretty good shape.
STINCHCOMBE: Yes, I think this interval is both prognostic and predictive of some of the benefit. And so I echo what other people have said: They’ve had a robust response to platinum, then robust response to the second-line therapy. You even start to wonder, is this SCLC, because if they do well, it makes you question the diagnosis.
We have clinical trials for this. It can be challenging to get this third-line population on a clinical trial, between the organ dysfunction, the performance status, and other aspects. So I think this is definitely an unmet need in a challenging patient population.

OSEI-BOATENG: An area I struggle with is selecting the best choice for those with brain metastasis.
KATRAGADDA: I agree with the brain metastasis and the myelosuppression. Going back to that, has anybody had experience using that CDK4 inhibitor with the carboplatin, or platinum regimen? It’s been approved for a while now, I think.5
STINCHCOMBE: Trilaciclib [Cosela].
MOHAMED: Yes, I have used it a lot. It’s already built into our care plan. Dr Katragadda and I are on the same system, so it’s available for us to use. I have used it in around 10 or 11 patients. Actually, I’ve got good results, so that’s reassuring.
RABARA: I recently started using it; so far, I only have 2 patients on it. And so far, I haven’t seen much neutropenia since I started using it first line.
MADADI: I have used it, too. I think neutropenia is helped by pegfilgrastim [Neulasta], but it also helps with the thrombocytopenia, which sometimes can lead to a lot of dose reductions. And using it is definitely being a protection for myelosuppression, and reducing the thrombocytopenia has been helpful, too.
STINCHCOMBE: What emerging studies are you most interested in, in ES-SCLC?
MOHAMED: I will be interested in anything that can work in SCLC. It has been a very challenging disease for the last 25 to 30 years, unfortunately. The progress is very mild and slight. Immunotherapy definitely is a great advance. I hope that we can build on that.
STINCHCOMBE: I think some of the trials that are in development. I’m not promoting this trial—we don’t have it at Duke—but Amgen has a bispecific T-cell engager trial [NCT03319940]. It’s opened at [Winship Cancer Institute at] Emory University, which might be an option for that, and we’re trying to get it at Duke in the future. This has shown a response rate in refractory patient populations.6 It does have the cytokine release syndrome, but it’s only on the first cycle.
We do have an antibody-drug conjugate. About 50% of people express the protein so we need either an old or new biopsy. I think there’s some interest in liposomal irinotecan; that’s another trial [NCT03088813] that’s going on globally, building on Dr Morse’s and others’ love for irinotecan. But the idea is that liposomal drugs will have better release, better pharmacokinetics, and may have more efficacy.
So I think those are some of the agents that are in development right now, for ES-SCLC, that we’re trying to keep an eye on. Then I think there’s some additional trials looking at different sequencing questions, potentially different timing of lurbinectedin in the maintenance setting. There’s the phase 3 trial [SKYSCRAPER-02; NCT04256421] of carboplatin/etoposide/atezolizumab with or without a T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains drug, and that’s a phase 3 trial that would look at a 4-drug immunotherapy vs carboplatin/etoposide/atezolizumab regimen.
REFERENCES
1. Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645-654. doi:10.1016/S1470-2045(20)30068-1
2. NCCN. Clinical Practice Guidelines in Oncology. Small cell lung cancer, version 2.2021. Accessed June 9, 2022. https://bit.ly/2JTv4zC
3. Hycamtin. Prescribing information. Novartis; 2018. Accessed June 9, 2022. https://bit.ly/3ttjSjL
4. Zepzelca. Prescribing information. Jazz Pharmaceuticals; 2020. Accessed June 9, 2022. https://bit.ly/3NFwtrY
5. FDA approves drug to reduce bone marrow suppression caused by chemotherapy. FDA. February 12, 2021. Accessed June 9, 2022. https://bit.ly/3970oKC
6. Owonikoko TK, Champiat S, Johnson ML, et al. Updated results from a phase 1 study of AMG 757, a half-life extended bispecific T-cell engager (BiTE) immuno-oncology therapy against delta-like ligand 3 (DLL3), in small cell lung cancer (SCLC). J Clin Oncol. 2021;39(suppl 15):8510. doi:10.1200/JCO.2021.39.15_suppl.8510

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More