Roundtable Discussion: Wagner-Johnson Highlights New and Emerging Approaches for DLBCL
Experts discuss the newest approaches for treating patients with diffuse large B-cell lymphoma.


WAGNER-JOHNSON: I would be interested to hear what is driving your decisions [in a patient case like this]. Other than the general gestalt of the patient, are there certain things that push you one way or another?
PROOTHI: Well, the eligibility for allogenic transplant is one [factor]; eligibility for CAR [chimeric antigen receptor] T-cell therapy is another. Then [I consider] the safety and tolerability of the regimen, comorbidities, patient preference, logistics—I think every item matters.
WAGNER-JOHNSON: Yes, it is sort of a combination of things. If you have a patient for whom you would consider CAR T-cell therapy, what things do you try to avoid as your second-line regimen?
EFIOM-EKAHA: One of the things [to consider] in terms of second-line therapy is the timing of the relapse, right? I think in [cases of] relapse within 12 months, the outcomes are better with CAR T-cell therapy, and maybe allogeneic transplant would be reasonable for someone who has relapse 5 years out from the initial therapy.
As far as the salvage therapy, if I am thinking about doing CAR T-cell therapy, I want to avoid therapies that [cause] lymphodepletion, as things like bendamustine [Bendeka] probably would not be part of that salvage regimen.
WAGNER-JOHNSON: Do others try to avoid bendamustine in the second-line setting for this reason?
NATHAN: Yes, I would avoid bendamustine for this reason.
EFIOM-EKAHA: Another thought would be [to consider, for example, that] you just, logistically, could not get a patient to CAR T-cell therapy early, [even though] that [might have been] your intention.
Should I also avoid CD19-directed therapies in that setting? Though I know for a fact that CAR T-cell therapy would still work [in the future] if I used loncastuximab [Zynlonta] or tafasitamab [Monjuvi] and [the patient] had a nice response. I do not know the answer to that.
WAGNER-JOHNSON: [The answer to that question] may reflect [whether the respondent is] from an academic center where they are going to keep [rather than refer] their patients, [and it may also reflect] patient preference and [the overall] patient population. I have heard from people in the community that some of their patients do not have any desire to come and see us [if they are referred here].
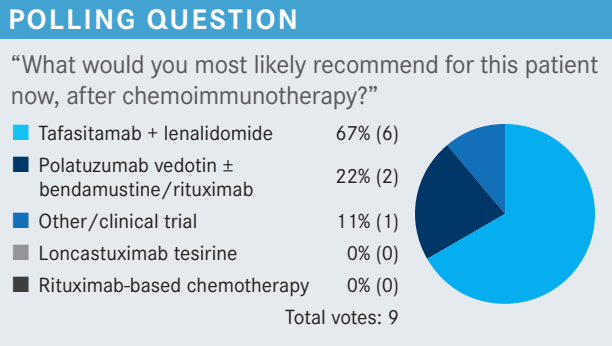
FAROUN: This patient had disease that was refractory to rituximab, and he relapsed 5 months after secondline therapy with rituximab-based therapy. In my opinion, [rituximab]-based therapy is not an option here. That leaves us with [loncastuximab], or [tafasitamab plus lenalidomide]. I think, because this patient has germinal cell subtype, lenalidomide probably is active in this setting, so I would choose tafasitamab plus lenalidomide, with dose adjustment.

WAGNER-JOHNSON: Some of you definitely have some experience with these regimens. [There is not as much experience with] loncastuximab, if I remember correctly; that was the newer one to be approved.1-3
RIZVI: Polatuzumab, in my experience, has been very well tolerated. Generally, for people who are not eligible for transplant or [for] any other treatment option, I have found polatuzumab to be pretty effective and pretty well tolerated.
WAGNER-JOHNSON: When you are giving it, do you typically give it with the BR [bendamustine/rituximab] or without?
RIZVI: These are patients who are very frail, so I end up giving [polatuzumab] by itself.
WAGNER-JOHNSON: Got it—I have not given [polatuzumab] singly yet. I often have given it as a bridge for my patients [when they] have had their CAR T cells collected and we are waiting for manufacturing, and I will frequently give, at that point, bendamustine.
At that point, the patients have already had their cells collected, so I am not as concerned, and [these patients] are typically more fit because they are heading to CAR T-cell therapy, and if I need to give bridging, they have [aggressive] disease. But I have not given [polatuzumab] singly yet.
I am pretty sure Dr Gladstone has some experience in that regard. Do you frequently give it [as part of a combination] or not? What is your experience?
GLADSTONE: I always [anguish] about it. Also, when I do use it, [my choice] depends on the disease burden. [That is, it depends on whether] I think I am going to get the patient to collection easily, or [I think] it is going to be very hard because [their disease is] so bulky. I know that does not answer the question, but that [is what] goes into my thinking, even though it is a non sequitur.
WAGNER-JOHNSON: [I see.] Dr Rizvi, you have had good experience [with polatuzumab] as a single agent, per the National Comprehensive Care Network [NCCN] guidelines, [which] clearly indicate [that option].4 Who else has had some experience with this regimen and wants to give their thoughts on choosing to give [polatuzumab] with or without the BR?
PROOTHI: I have used it with the rituximab [only], without the bendamustine. Obviously, if [the patient] had a neurotoxicity a year before, then I would not pick the polatuzumab. If [the patient] has a high-risk [status after] transplant, I would rather go with the loncastuximab; otherwise, I would not have any preference.
The other question I have is, for the tafasitamab plus lenalidomide combination, if the [lenalidomide] dose matters, at 25 mg,5and if [the patient] gets grade 4 neutropenia, should I use the growth factor and [maintain] the dose or should I lower the dose?
WAGNER-JOHNSON: I have given growth factors successfully and have been able to maintain [the dose]. I think it frequently happens that [the patient’s neutrophil count] is so, so low that you must [stop treatment] for a bit or lower [the dose]. I usually do not have the luxury of catching [the neutropenia] early, [such that] I can give the growth factor and avoid problems. Often, I do both simultaneously, [and when] I give the growth factor, the counts recover, and then I consider escalating.
I think part of the problem is the logistics of [finding the appropriate] dose for the patient because [every adjustment] requires a new prescription. But I do often try to push [the dose] back up. I have not had as much luck when giving that with just rituximab, and I need to gain some more experience with [tafasitamab].
FAROUN: I am hopeful that, soon, polatuzumab is going to move to frontline therapy for [patients with] DLBCL. I think it is not going to be used in the second line or beyond. So we [will be] left with 2 regimens: loncastuximab [and the combination of] tafasitamab and lenalidomide.
I think if the patient is refractory to rituximab or has germinal centers, we will use tafasitamab plus lenalidomide. I do not have any experience with loncastuximab yet but [I am hopeful that] we can use it in the future, but again, if polatuzumab is moving up [to the first line], I think that will leave us with 2 options only.
WAGNER-JOHNSON: That is a good point. Could we hear from a few more people about their thoughts in terms of which [option] seems the most appealing, or [in terms of] the perfect fit for a particular drug?
PROOTHI: [Regarding] polatuzumab, some centers are taking vincristine out of R-CHOP [and replacing it with] polatuzumab instead.6
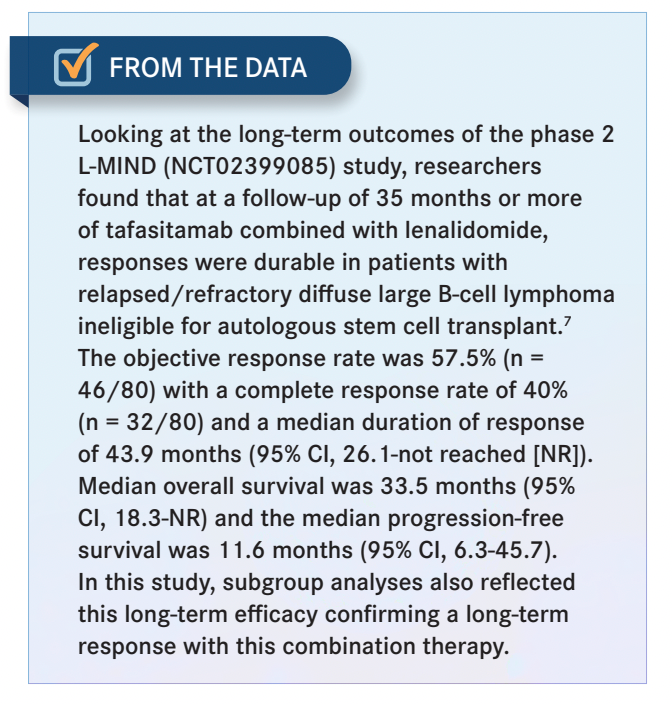
EFIOM-EKAHA: My concern is that in the community, a lot of times, we want to salvage these patients with CAR T-cell therapy, but logistically, sometimes [it is] a little tricky to get them to CAR T-cell therapy, and so we have inertia. We say that we do not want to use a CD19-targeted drug [because] we do not want to burn the bridge, but while you are trying all kinds of bridging therapies, the disease could be progressing. I [think that] tafasitamab plus lenalidomide, with a 40% complete response [CR] rate and almost a 60% overall response rate,7 is a great regimen [From the Data7].
So I say to myself, why don’t I just use my most active therapy and worry about the logistics of CAR T-cell therapy later rather than be afraid of using a CD19-targeted therapy [just] because I think [the patient] may get to CAR T-cell therapy [eventually]?
GLADSTONE: I believe it is a true statement that the tafasitamab molecule binds to a different section of CD19. But the concern that tafasitamab is going to outcompete [rituximab is] probably not going to be borne out in the future.
WAGNER-JOHNSON: Yes, and we [have not talked] about bispecific T-cell engager [BiTE] therapies; none of those are currently approved but they look incredibly promising.
It is going to be interesting to see [how things develop with respect to] naked antibodies, antibody-drug conjugates [ADCs], BiTEs, and CAR T-cell therapies. There is going to be a breadth of available treatments.
And from a practical standpoint, if our ADCs compare relatively well with a BiTE, my guess is that most in the community will be more comfortable choosing the ADC. Similarly, [we will see] whether the BiTEs, especially in combination with other agents, hold up in competition with CAR T-cell therapies. Of course, we do not have mature data to make these statements, but I think it is interesting to look at [the] CR rates [because] they are impressive. I think a lot is going to be learned about these novel agents, and where they fit in, with further follow-up in the next few years.
GLADSTONE: The thing that is impressive, I think, is that tafasitamab by itself does not get a [grade of] A-plus. Tafasitamab plus lenalidomide is much better than tafasitamab alone, and for patients who are chemotherapy resistant, [it is important to note that] bendamustine is chemotherapy, and loncastuximab is [not]. What is nice about the combination of tafasitamab and lenalidomide [is that] it is purely immunologic. So if you think a patient’s cancer cells do not have the ability to respond to a chemotherapy signal, tafasitamab plus lenalidomide does not use chemotherapy.
WAGNER-JOHNSON: True.
GAFFAR: I did use loncastuximab recently for a patient with horrible renal sufficiency and relapsed DLBCL. I did use tafasitamab plus lenalidomide, and then recently I put him on loncastuximab, and he has had a good response so far. As an aside, he did have a repeat biopsy; he has gingival involvement, so they [easily biopsied] it and found that it was still CD19 positive.
WAGNER-JOHNSON: Good. [Were there] any problems with the unique toxicities that we are seeing, edema or elevation of liver enzymes?
GAFFAR: Not really. Maybe a little bit of edema but nothing else.

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More