Burger Details the Data to Help Choose Treatment in Chronic Lymphocytic Leukemia
During a Targeted Oncology case-based roundtable event, Jan A. Burger, MD, PhD, discussed data supporting the use of ibrutinib, acalabrutinib, and zanubrutinib for first-line treatment of chronic lymphocytic leukemia.
Jan A. Burger, MD, PhD
Professor, Department of Leukemia
Division of Cancer Medicine
The University of Texas MD Anderson Cancer Center
Houston, TX

Targeted OncologyTM: What do the National Comprehensive Cancer Network (NCCN) guidelines recommend for a patient such as this?
BURGER: The most recent NCCN guidelines recommend the Bruton tyrosine kinase [BTK] inhibitors acalabrutinib [(Calquence) with or without obinutuzumab (Gazyva)], ibrutinib [Imbruvica], and venetoclax [Venclexta] with or without obinutuzumab, [which are] all supported by category 1 data. Zanubrutinib [Brukinsa] is listed also, but that is not FDA approved yet [for CLL], but may be approved later this year or next.1
The chemoimmunotherapy approaches, [once listed as “preferred regimens”], are now listed as “other recommended regimens.” That’s the result of many randomized studies that demonstrated better progression-free survival [PFS] and, in some studies, better overall survival [OS] with the new targeted agents, which are also better tolerated.
What data support the use of first-line ibrutinib for patients with CLL?
The study that put ibrutinib on the landscape was the phase 3 RESONATE-2 study [NCT01722487], which started around 2012 or 2013. At that time, chlorambucil [Leukeran] monotherapy was still considered a standard treatment, so patients were randomly assigned to receive either ibrutinib or chlorambucil. This study was for older patients, [at least age 65 years], who had comorbidities.2,3
We have followed these patients for 5 years or more, and we published and presented those data last year. There was a major difference in PFS from the beginning, favoring ibrutinib, but there was some decline [in PFS even in the experimental arm], which I think was mostly driven by the development of adverse events [AEs] rather than resistance to ibrutinib.
[Patients who develop AEs] eventually must come off treatment and then progress. But the data clearly show that targeted therapy works much better than chlorambucil at median PFS for the experimental arm vs the comparator arm, that was not reached (NR) vs 15 months, respectively [HR, 0.160; 95% CI, 0.111-0.230].4 Similar studies like [iLLUMINATE (NCT02264574) and ELEVATE-TN (NCT02475681)] have been done with chlorambucil plus obinutuzumab as a comparator arm, and the data looked quite similar.
When we talk about favorable vs unfavorable [status], or high-[risk] vs low-risk CLL, those terms are helpful for predicting the time until the patient may require treatment. The treatment outcome for the formerly high-risk, [IGHV]- unmutated patients [HR, 0.109; 95% CI, 0.063-0.189], was basically identical to that of the low-risk, [IGHV-mutated] patients [HR, 0.197; 95% CI, 0.096-0.394]. So, in terms of treatment outcome, the prognosis has much improved, and these high-risk patients have durable responses similar to those of their counterparts in the low-risk category at [6.5-year PFS, 62% vs 67%, respectively].
[In contrast, the results for the chemotherapy arm were] what we are used to: [IGHV-]mutated patients fared better than the [IGHV-]unmutated patients [6.5-year PFS rate, 18% vs 2%, respectively].4
Five-year OS rate was not very different between the arms [83% in the experimental arm vs 68% in the comparator arm]. [Median OS was NR in both arms (HR, 0.514; 95% CI, 0.312- 0.848)]. The difference in OS would be greater if there hadn’t been crossover, but patients were allowed to cross over if they had disease progression after 12 months.4 That rescued some of these patients; obviously, if a patient progresses, you put them on other, [more] effective treatment.
What did the data reveal about the safety of this regimen?
There were not many serious AEs. In the experimental arm, there were some cases of pneumonia [4%] and some basal cell cancers [2.2%], which we often see in our patients with CLL. The AEs of clinical interest [included atrial fibrillation]; this was one of the first studies to point out that there is a risk for atrial fibrillation in patients who receive ibrutinib and less risk with chlorambucil. In this study, atrial fibrillation was observed in 6% vs 0.8% of the patients in the respective arms.2 That’s [approximately] 1 in 20 patients. That doesn’t necessarily mean the patients cannot continue treatment but obviously it is something you must make your patients aware of if you use BTK inhibitors, [especially] ibrutinib.
Hypertension was a more common cardiovascular AE, [affecting 14% of patients in the experimental arm vs 0% in the comparator arm].2 There was also a slight risk of increased bleeding, [affecting 4% vs 2%, respectively]. All the BTK inhibitors have that effect because they affect platelet aggregation. If you have patients on anticoagulation therapy, you must watch them closely for bleeding events, especially when you initiate treatment, and you must hold treatment [if they] undergo any type of procedure where they could potentially bleed.5
After 7 years, about half of the patients are still on treatment [47%], but some patients had to discontinue treatment, mostly because of AEs [23%]. Other patients [discontinued treatment because of] disease progression [12%] or for other reasons.4
In this study, we are talking about older patients, so they were taking other medications. Additionally, many of the patients were on acid-reducing medications; more than half were on proton pump inhibitors [with 56% in the experimental arm vs 36% in the comparator arm, respectively]. These results tell you that you can safely [administer ibrutinib to] patients who are on these different medications if you monitor them carefully.4
What data support the use of acalabrutinib as first-line treatment for CLL?
The phase 3 ELEVATE TN trial looked at acalabrutinib, the more recent, second-generation BTK inhibitor, which is a bit more selective for BTK. It inhibits [fewer] off-target kinases and the hope is that it therefore has a [less severe AE profile], and there are some data now to indicate that there are fewer cardiovascular AEs. This study was done in treatment-naive patients, divided among 3 arms: acalabrutinib plus obinutuzumab, single-agent acalabrutinib, and obinutuzumab plus chlorambucil.6
PFS was best for patients in the acalabrutinib plus obinutuzumab arm. There was a slight PFS benefit from adding obinutuzumab to acalabrutinib, but not very much. [Median PFS was NR in both arms at a median follow-up of 46.9 months (HR, 0.56; 95% CI, 0.32-0.95; P = .0296)], and you have more AEs once you add obinutuzumab. The chlorambucil plus obinutuzumab arm was inferior, [with a median PFS of 27.8 months]. [For acalabrutinib plus obinutuzumab vs chlorambucil plus obinutuzumab, the HR was 0.10 (95% CI, 0.07-0.17; P < .0001)]. So, their conclusion was in favor of single-agent acalabrutinib, [which produced 78% PFS at 4 years]. Clearly, consistent with all the BTK inhibitor studies, this study had a very good outcome.6,7
When the results were analyzed regarding mutation status, the survival curves were very similar; patients with unmutated IGHV and those with del(17p) or mutated TP53 were doing well [median PFS, NR]. I think the message here is that the BTK inhibitors ibrutinib and acalabrutinib work very well in low-risk and high-risk patients.4,6,7
OS looked very good [after a median follow-up of 46.9 months], even for the control arm, because patients could move on to a very effective treatment. [Median OS was NR for all arms; survival rates at 4 years was 93% in the acalabrutinib plus obinutuzumab arm, 88% in the acalabrutinib arm, and 88% in the chlorambucil plus obinutuzumab arm.]6
What did the data reveal about the safety of this regimen?
In terms of safety, there’s not too much to point out. The most common AEs were [anemia, neutropenia, upper respiratory tract infection (URTI), thrombocytopenia, headache, musculoskeletal pain, and] diarrhea. The headaches are responsive to anything containing caffeine; they are usually not very severe and go away within the first couple of months. Overall, this is a very well-tolerated drug. Atrial fibrillation of any grade occurred [in 3.9%, 3.4%, and 0.6% of the patients in the acalabrutinib, acalabrutinib plus obinutuzumab, and chlorambucil plus obinutuzumab arms, respectively].
For hypertension, [the values were 4.5%, 7.3%, and 3.6%, respectively]. There were numerous bleeding events, [affecting 39.1%, 42.7%, and 11.8% of patients in the respective arms]; this was mostly bruising, as physicians will see in their ibrutinib-[treated] and acalabrutinib-treated patients. Some patients tend to bruise more easily, especially if they aren’t on additional anticoagulation or antiplatelet agents. The occurrence of secondary malignancies, [which affected 2.8%, 5.6%, and 1.8% of patients in the respective arms], were probably just a result of patients being on long-term treatment and having some risk for other cancers.6,8
What data support first-line use of zanubrutinib?
Zanubrutinib, a new, second-generation BTK inhibitor, is not FDA-approved for CLL. It is approved for mantle cell lymphoma and for Waldenström macroglobulinemia.9,10 This drug was studied in the SEQUOIA trial [NCT03336333]. There were 3 cohorts in this trial; the data from cohort 1 reflect frontline treatment of CLL patients who received either zanubrutinib or bendamustine [Bendeka] plus rituximab [Rituxan]. The patient population was of a representative age, about 70 years, and [the patients did not have detectable del(17p)], so it was a very typical population of older frontline patients.11
PFS was analyzed and, as in [most of] the studies comparing BTK inhibitors to chemoimmunotherapy, there was a significant benefit from the new BTK inhibitor; [24-month PFS rate was 85.5% (95% CI, 80.1%-89.6%) vs 69.5% (95% CI, 62.4%-75.5%) for the experimental and comparator arms, respectively]. The IGHV-unmutated patients did better with zanubrutinib than with the comparator combination [HR, 0.24; 95% CI, 0.13-0.43; P < .001], [with results similar to those observed with ibrutinib].4,11 Additionally, the low-risk, mutated patients did OK with bendamustine plus rituximab [HR for zanubrutinib vs bendamustine plus rituximab, 0.67; 95% CI, 0.36-1.22; P = .186] and they certainly did better than the high-risk, [unmutated patients who received that combination].11 The overall response rate [ORR] was good. With the BTK inhibitors, you can’t expect to see a lot of complete remissions, but the ORR was high [94.6% (95% CI, 91.0%-97.1%) for the experimental arm vs 85.3% (95% CI, 80.1%-89.5%) for the comparator arm].11
It is exceedingly rare for a patient with CLL not to respond to a BTK inhibitor. If the patient does not respond, you must question your diagnosis [and consider whether] the patient may not have some other problem or maybe a different malignancy.
What did this study reveal about the safety profile of this drug?
Overall, zanubrutinib appeared to be a well-tolerated drug. There were some AEs in the zanubrutinib arm, but fewer than in the bendamustine plus rituximab arm; [AEs of grade 3 or higher affected 52.5% vs 79.7% of patients in the respective arms (Table11)]. Dose reductions occurred sometimes, [in 7.5% vs 37.4% of the respective arms]. Also, the rate of discontinuation due to AEs was not very high, [8.3% vs 13.7%, respectively], although the follow-up was relatively short, [approximately 26 months].11 [The AEs that occurred most in this study are similar to what] you’ve probably seen with other BTK inhibitors. These included arthralgia, [observed in 13.3% of patients in the experimental arm vs 8.8% of patients in the comparator arm].
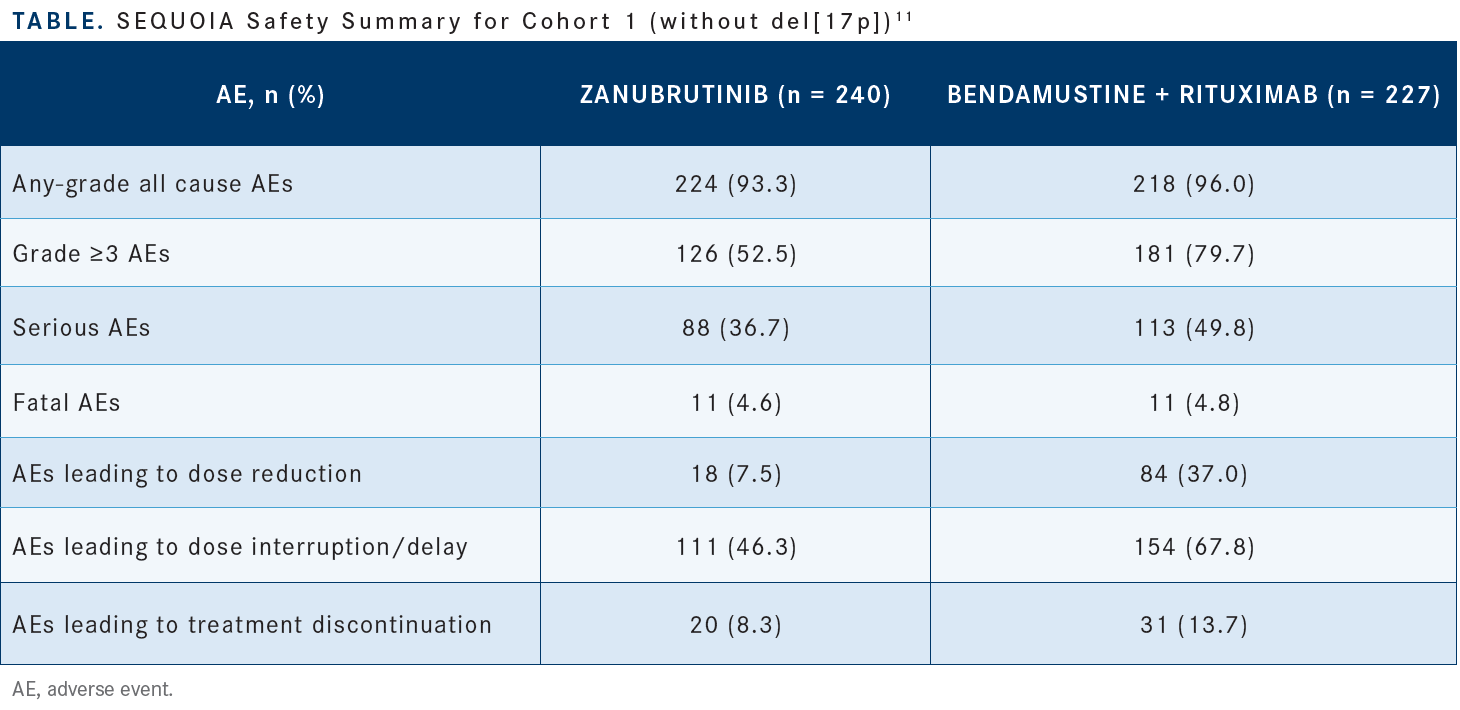
These joint aches can bother patients, especially in the beginning of treatment. Some patients had URTIs, less with the BTK inhibitor [17.1%] than with chemoimmunotherapy [11.9%]. Myelosuppression is rare with the BTK inhibitors, [and in this study, neutropenia affected 15.8% vs 56.8% of the respective arms]. Infusion reactions occurred among the patients who received rituximab, but obviously none were observed among patients who received the oral agent.
In this study, among AEs of interest, there was certainly more myelotoxicity with the comparator than with zanubrutinib. There was a little bit of neutropenia in the zanubrutinib arm, [affecting 15.8% of patients], which is not seen with acalabrutinib or ibrutinib. Dose reduction should fix that problem.
Atrial fibrillation was not very frequent, affecting only 3% of the patients in the experimental arm [vs 2.6% in the comparator arm], but that’s with a relatively short follow-up. Bleeding of any grade affected 45.0% [vs 11%, respectively], but major bleeding was not very frequent. There was some diarrhea [13.8% vs 13.7%, respectively] and some hypertension [14.2% vs 10.6%], but, overall, this was another well-tolerated drug.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Chronic lymphocytic leukemia/ small lymphocytic lymphoma, version 3.2022. Accessed June 3, 2022. https:// bit.ly/3xxeWMY
2. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. doi:10.1056/NEJMoa1509388
3. Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. doi:10.1038/ s41375-019-0602-x
4. Barr PM, Owen C, Robak T, et al. Up to seven years of follow-up in the RESONATE-2 study of first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. J Clin Oncol. 2021;39(suppl 15):7523. doi:10.1200/JCO.2021.39.15_suppl.7523
5. Imbruvica. Prescribing information. Pharmacyclics; 2022. Accessed June 6, 2022. https://bit.ly/3xyGW2U
6. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab vs chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
7. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Elevate-TN four-year follow up. J Clin Oncol. 2021;39(suppl 15):7509. doi:10.1200/ JCO.2021.39.15_suppl.7509
8. Calquence. Prescribing information. AstraZeneca; 2019. Accessed June 6, 2022. https://bit.ly/2kdeWv5
9. FDA grants accelerated approval to zanubrutinib for mantle cell lymphoma. FDA. Updated November 15, 2019. Accessed June 6, 2022. https://bit.ly/39bd0AI
10. FDA approves zanubrutinib for Waldenström’s macroglobulinemia. FDA. Updated September 9, 2021. Accessed June 6, 2022. https://bit.ly/3xdOeYB
11. Tam CS, Giannopoulos K, Jurczak W, et al. SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab (BR) in patients with treatment-naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood. 2021;138(suppl 1):396. doi:10.1182/blood-2021-148457

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More