Roundtable Discussion: Barata Explores Second- and Third-Line Options for Recurrent RCC
During a Targeted Oncology case-based roundtable event, Pedro Barata, MD, MSc, discussed the case of a patient with clear cell renal cell carcinoma who progressed following frontline treatment with axitinib/pembrolizumab.

Pedro C. Barata, MD, MSc (Moderator)
Assistant Professor of Medicine
Section of Hematology and Medical Oncology
Tulane University School of Medicine
Tulane Comprehensive Cancer Clinic
New Orleans, LA


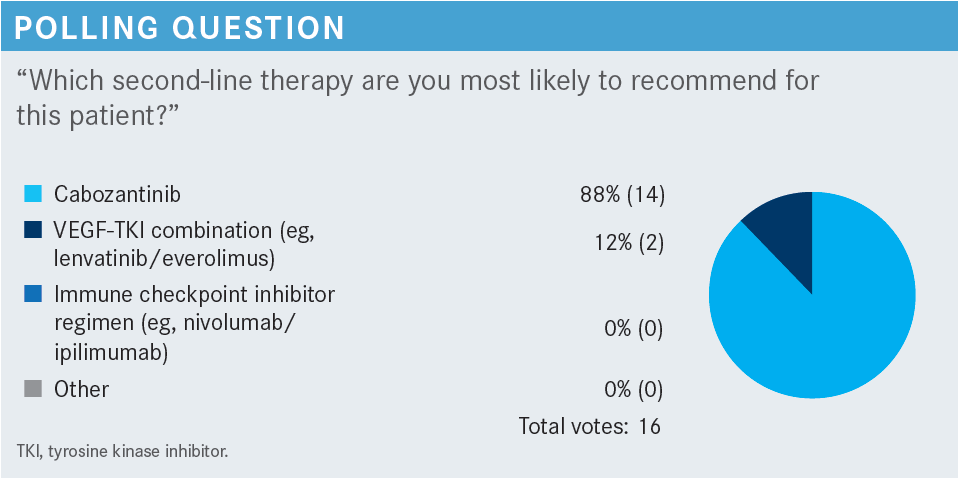

BARATA: We have a number of factors such as tolerability, toxicity, efficacy, patient preference, guidelines, and type of disease. What would you highlight at this point for considering one therapy vs another? Let us say he [still] has an ECOG performance score of 1.
MULHERIN: How symptomatic is the patient?
BARATA: Let’s say he is not [very symptomatic]. He is having some weight loss, but not a lot. His tolerability to cabozantinib was OK, a bit of diarrhea, but manageable. He has a bit more fatigue, but not that different from when he was on axitinib 5 months ago. How does this affect your choice?
MULHERIN: I think we could do lenvatinib [Lenvima] plus everolimus [Afinitor] or tivozanib [Fotivda], either of those would be possibilities. I have used both. I have used tivozanib in a couple of patients who made it that far and were still candidates for treatment. I have not had as many good experiences with everolimus. I know you can mitigate the stomatitis with oral dexamethasone, but I have found that it is not as easy [to tolerate].
BARATA: Is it everolimus monotherapy or lenvatinib plus everolimus you are referring to?
MULHERIN: I was thinking about putting them together. If you were going to send them for a clinical trial, he has an ECOG performance score of 1, so he would be a great option for a 2 vs 1 trial. But if I am not going to do that, it would be 1 of those 2.
With an ECOG performance score of 1, he should be able to tolerate the toxicities of either of those regimens. I would be more inclined to do lenvatinib plus everolimus because he would be getting a totally different mechanism of action with an mTOR inhibitor and, ideally, he should be able to tolerate that.
CIOBANU: I agree with Dr Mulherin. The only thing that I was thinking is that the response rate of tivozanib is not great, I mean it is [23%] and this patient got only 4 months of response to cabozantinib [From The Data1]. I do think that in this situation, he is already exposed to a third-generation TKI [tyrosine kinase inhibitor] with axitinib, so probably the best choice for him in terms of a response would be lenvatinib with everolimus because it has a better response rate.2
We do not know the response rate as third-line therapy, but it has a different mechanism of action. As Dr Mulherin mentioned, [lenvatinib plus everolimus] is not an easy regimen to tolerate, so it is unlikely that it will be tolerated better later in the disease course. I think this may be the time to choose it.
BARATA: Among the different end points, do you favor response rate over measures such as progression-free survival [PFS] or overall survival? Is that your go-to end point when you are thinking of the refractory space, along with quality of life [QOL]?
CIOBANU: Yes, response rate [is my favored end point].
BARATA: Would you be concerned about the tolerability?
CIOBANU: If a patient is very symptomatic, [I’d be concerned;] obviously, you want somebody to have a higher chance to respond. But [I’d also like] a longer duration of response. He had only 4 months PFS on cabozantinib. You want something better, and a chance of PFS as well.
BARATA: That is fair. Dr Konala, do you agree or do you prioritize other things? Is this combination regimen what comes to mind in this setting? What is the goal of therapy? Do you care about quality of life [QOL]? Do you care about a patient not progressing and tolerating the regimen well?
KONALA: I agree with the prior speakers. Axitinib was tried and tivozanib is [similar]. I would go with lenvatinib and everolimus. If the patient is symptomatic, I would go with response rate because this combination has the highest response rate [of 43%].2 They tried axitinib and cabozantinib, but lenvatinib targets a slightly different receptor compared with cabozantinib. Everolimus is an mTOR inhibitor, so it has a different mechanism of action.
I have tried this therapy only in 2 patients so far, but it is hard to tolerate. I had to dose reduce [because] I had patients with hypertension and diarrhea. I have used it in very young patients with translocation RCC and they did not respond much to it. However, in the third line, I think it is the best option we have with a different mechanism of action and different receptors being targeted.
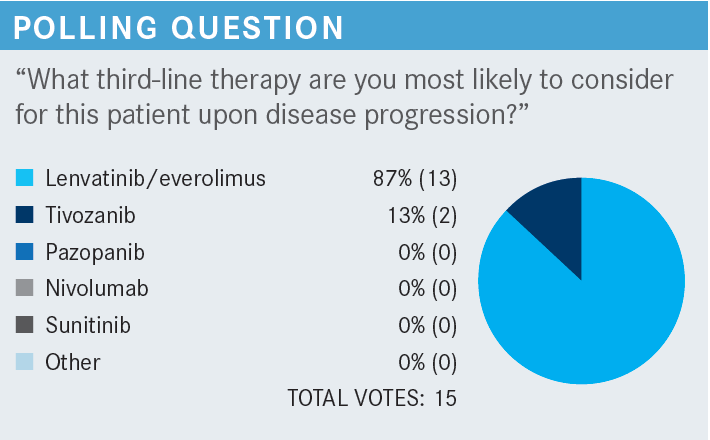
BARATA: Dr Baldeo, what would be your thoughts about lenvatinib plus everolimus vs tivozanib?
BALDEO: I have not used tivozanib, but based on the trial, the adverse effects [AEs] look comparable. I have used lenvatinib plus everolimus, and I have always had to dose reduce mainly because of the fatigue and diarrhea.
BARATA: Based on the data, do they favor tivozanib, or not so much?
BALDEO: Well, the dose reduction favors tivozanib because I think [the number of patients needing dose reductions] was more than 70% with the everolimus plus lenvatinib arm.2 I am not familiar with using it, but I would be willing to give it a try just because it has [fewer] dose reductions, based on the trial.3
BARATA: Dr Oppelt, any other thoughts? Of course, we do not have any head-to-head studies. What are your thoughts about putting things into perspective? What is your clinical judgment of the data?
OPPELT: I would echo a lot of what has been said. I think the data are impressive in that it does look like it is a well-tolerated drug in the third-line setting. I think a lot of patients are interested in that. As far as its efficacy, it is impressive that there are patients who are 2 to 4 years out without evidence of progression.4 I think it is a reasonable option in terms of being aggressive against the disease compared with the lenvatinib and everolimus combination.
BARATA: Dr Bendaly, any comments on the toxicities? Would you feel comfortable with one vs the other?
BENDALY: I am one of the few oncologists not aware of the tivozanib data. I picked lenvatinib plus everolimus in the third-line setting, but after what you shared with us, I think tivozanib would be my go-to third-line agent.
My thought process initially was that [tivozanib] has the same activity profile against all the different TKIs as axitinib and we had used axitinib earlier for this patient. So it would not overcome the resistance, especially because both are third-generation TKIs, but the data are compelling. In terms of managing hypertension as the main AE, I think it is not a big drawback.

BARATA: Do you think of frontline therapy first and then see what happens? Do you think about what is going to happen in the second-line and third-line settings?
BENDALY: I think about it from the start. I try to use immuno-oncology [IO] drugs up front and keep TKI options for later. If we use pembrolizumab plus axitinib in the front line, and the patient progresses, we have burned the IO and TKI options for the time being. I understand that some providers might come back and use an IO in later lines. [Based on] the data, and from my experience with this combination for the higher-risk patients, [I know] some patients end up doing quite well for a very long time. I have not seen this with axitinib and pembrolizumab. Full disclosure, I have not used pembrolizumab and axitinib for as long as I have used the double IO [nivolumab (Opdivo) plus ipilimumab (Yervoy)], probably because they have not been on the market for as long.
BARATA: Are these important data for you to think of post IO, post checkpoint inhibitor, post TKI? What are your thoughts on if that will affect your use of tivozanib in this setting, Dr Spigel? Are these data enough for you to think [of going] with a TKI vs an mTOR such as everolimus?
SPIGEL: I think the data are compelling. I think tivozanib is a good option, but the comparator arm is what troubles me. Sorafenib [Nexavar] is something that feels a little bit antiquated and maybe not the best comparator arm. I like the comments that have been made about switching mechanisms of action. It is just that my experience with everolimus and mTORs in general has been modest. [I view them as] drugs that have a bit of toxicity and not a lot of responses.
I think we are stuck. This is a [case] where you wonder if they got a fair shake with the IO, but you can’t push it because of the pneumonitis. So you are stuck with the VEGF pathway and all the drugs…have overlapping mechanisms of action, and some are probably a little bit better than others. I just think we must keep trying them sequentially and try to squeeze as much out of them as we can, but I think the lenvatinib plus everolimus data are interesting.
BARATA: Does it bother you that the control arm is also everolimus? Although we have lenvatinib alone there too.
SPIGEL: Yes, that is a reasonable control arm in this group. For doublet therapy in a patient who is on the third line and has been on treatment this long, you know you are going to be dose modifying and dropping something quickly. There are not a lot of great options, and probably nothing is going to give you sustained disease control. So you start to think of QOL and try to minimize progression as best as you can.
BARATA: So [you would say that] the data are compelling, but QOL is the important goal in this setting. You want to control disease, but the patient should not be doing [poorly] while you do that. The goal is to buy time. Is that a fair summary?
SPIGEL: I would call Dr Garmezy first to ask for advice because I work with him. I do not [currently] treat much RCC. I treat a lot of lung cancer, but when I did treat a lot of RCC, I used a ton of bevacizumab [Avastin]. I liked it and it was easy, but I see it is a class [2B] recommendation now. Nobody uses it anymore?
BARATA: Very unlikely. To be honest with you, I also forget the last time I prescribed sunitinib [Sutent] and pazopanib [Votrient].
SPIGEL: Yes, fair enough.
BARATA: Things are changing. Any final thoughts on this, Dr Anwar?
ANWAR: PFS was 3.9 months for sorafenib vs 5.6 months for tivozanib.3 It is an absolute difference of 1.7 months. But the control arm was sorafenib, which is not an mTOR inhibitor. In your practice, in how many patients on third-line therapy do you use tivozanib vs lenvatinib or anything else?
BARATA: We are talking about a heavily pretreated population. [In the TIVO-3 trial (NCT02627963), 38% in the tivozanib arm and 41% in the sorafenib arm received] 3 lines of prior therapy, whereas [62% in the tivozanib arm and 59% in the sorafenib arm received] 2 lines of prior therapy.3 So you can see that anything you do will be more limited.
The goal here is not to cure. I do not think we are able to reasonably offer a cure to these patients. Even the response rate depends on how miserable they are going to be. I put things into context and [consider] the goal of care. To me, it is far more important to be without progression than [to be] looking at the response rate.
I do not want tumor shrinkage because it does not matter to me if you have a bunch of lung metastases shrinking 20% vs 30%. I do not think that is clinically significant for a patient in front of me in the third- or fourth-line setting.
The other piece of the story is a lot of these patients are getting IO in the second-line setting too. An mTOR is basically an immunosuppressive drug, and we do not have good data post IO of what happens with lenvatinib plus everolimus. None of these data are perfect, I have to say.
When you look at cabozantinib in the METEOR study [NCT01865747] or nivolumab in the CheckMate 025 study [NCT01668784], the data were for pre-IO agents in the frontline setting. Lenvatinib plus everolimus was pre-IO in the frontline setting too. What we do not know is the activity of that combination post IO. I like the fact that when you look at tivozanib, the data are more contemporary, which means more patients were exposed to a prior checkpoint inhibitor and you had a good number of patients who actually got there.
We know who got checkpoint inhibition there. I would like more data with axitinib and cabozantinib. For tivozanib you have data after checkpoint inhibition, which reflects more of what we do in clinical practice. [Concerning] safety, for my patients, lenvatinib plus everolimus is not [easy]. I do it, but we just need to be aware of the safety profile.
I am not going to tell you what we should do because I am not here to provide advice, but those are some of my thoughts and insights about what options are out there. Because I do not have a head-to-head study to definitively tell you A is better than B, I cannot make that assumption. I shared with you how I interpret the data, but your thoughts and insights are well taken.
GARMEZY: At the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium, patients who had axitinib and prior lines actually did get responses with tivozanib.5 That is telling you that this is an active post-VEGF inhibitor. A lot of those patients had 2 TKIs prior to tivozanib. So why is it working?
There has been a lot of focus on different mechanisms of action, but we do not actually know what is going on in the tumor microenvironment of those individual patients. There are some patients who have a more angiogenic phenotype, and others more of an immunologic phenotype.
If there is resistance through either upregulation of VEGF receptors or changes in the VEGF receptor vs different types of resistance mechanisms, you may be better off with tivozanib if you need that extra VEGF punch. The problem is we do not know at any given time what that patient would be best served by. That is the challenge. But I would just highlight that it is not always about switching mechanisms of action. That is important, though, because if you must dose reduce, all the data about benefit start to get a little bit murkier as to how your individual patient is going to do in the clinic.
Are you giving them enough of a TKI to augment that anti-mTOR therapy? That is just something that I keep in mind when I am thinking about my patients and explaining choices of therapy.
REFERENCES
1. Verzoni E, Escudier B, Hutson TE, et al. TIVO-3: durability of response and updated overall survival of tivozanib versus sorafenib in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2021;39(suppl 15):4546. doi:10.1200/ JCO.2021.39.15_suppl.4546
2. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473-1482. doi:10.1016/ S1470-2045(15)00290-9
3. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. doi:10.1016/ S1470-2045(19)30735-1
4. Pal SK, Escudier BJ, Atkins MB, et al. Final overall survival results from a phase 3 study to compare tivozanib to sorafenib as third- or fourth-line therapy in subjects with metastatic renal cell carcinoma. Eur Urol. 2020;78(6):783-785. doi:10.1016/j.eururo.2020.08.007
5. Rini BI, Pal SK, Escudier B, et al. TIVO-3: tivozanib in patients with advanced renal cell carcinoma (aRCC) who have progressed after treatment with axitinib. J Clin Oncol. 2021;39(suppl 6):278. doi:10.1200/JCO.2021.39.6_suppl.278

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen
Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More