Clinical Commentary: Addressing Challenges in Unresectable HCC and Adding Lenvatinib to Therapy Sequencing
During a Targeted Oncology case-based roundtable event, Ghassan K. Abou-Alfa, MD, discussed therapeutic sequencing for patients with unresectable hepatocellular carcinoma and the use of lenvatinib versus sorafenib.

Ghassan K. Abou-Alfa, MD
Medical Oncologist
Memorial Sloan Kettering Comprehensive Cancer Center
New York, NY

Serious AE Concerns in Certain Treatments
Interestingly, I would say that the concern about Crohn disease with infliximab [Remicade], and regarding checkpoint inhibitors, is valid.1 Maybe it’s not an absolute, but it’s valid, and if you decide to do that, you usually will send the patient to a gastroenterologist so that they assess the patient. They’ll tell you what kind of stability the patient has so you can get some sense about what you’re concerned about and if [you should stay] concerned about it. When it comes to the variceal bleeding [seen in patients], I would be more seriously concerned and even push [addressing it] further.2
Six months is at least my minimum regarding variceal bleeding to be banded without any activity because you can pull it out. As you can imagine, the variceal bleeding is like very high-pressure vessels that when they open in the esophagus they pour blood like there’s no tomorrow. I’m sure some of you might have the sad reality of seeing one of those gastrointestinal [GI] bleeds coming out of variceal bleeding in the emergency room. They are not a joke. I would say this is where I would not fiddle at all. If a patient had, 2 months ago, a variceal bleed, I would not give bevacizumab [Avastin].
Now, this brings up the question of what do we do? No 1, regarding the idea of giving atezolizumab [Tecentriq] by itself, I can understand [that thinking], but we must remember that atezolizumab by itself was never tested. We don’t know how much it can do or cannot do, and more importantly, one-third of the patients who get atezolizumab, without you knowing, will have an antibody against the drug that will make it ineffective. The question is: are we ready to take that risk without even testing, without knowing, without even knowing futuristically, even after you give it? I think we need to at least ask that question or have that discussion with our patients.
Then, we come to the choices of the tyrosine kinase inhibitors [TKIs]. I must admit, I would be rather nervous about sorafenib compared with lenvatinib. In my hands-on experience, I’ve seen GI bleeds on sorafenib, and I know they can happen on either of them, but I’m [more worried about them occurring for patients on sorafenib]. Frankly, I would say that the lenvatinib is way easier to manage.
Now, when sorafenib came on board, the sponsor tried to push for lower doses with the idea that maybe there would be less hand-foot syndrome. But I would say that starting at 12 mg, with the understanding that we can lower it if need be, is totally OK [Table3].
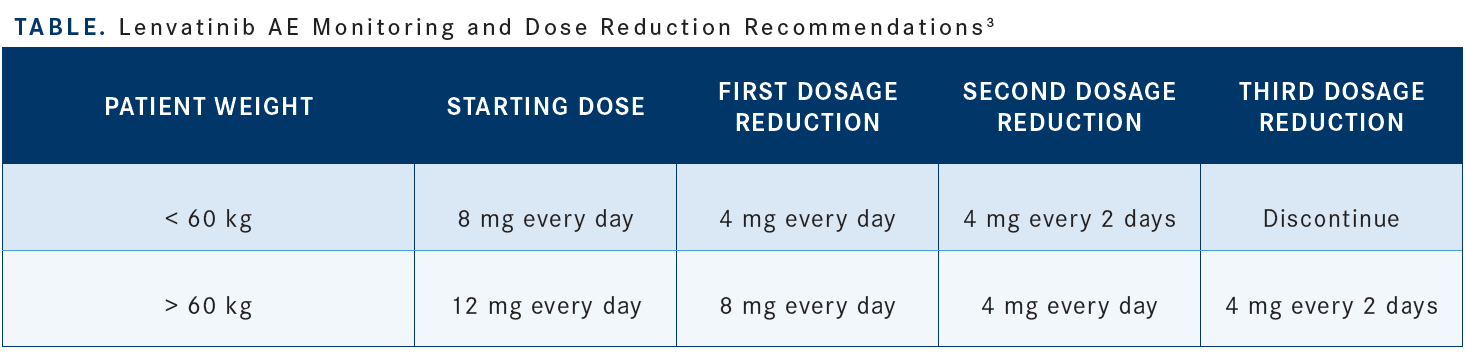
The most important thing, regardless, is to make sure that you see those patients. When I start somebody on lenvatinib or sorafenib, I’ll see them within a week because you have to keep an eye on them because things will declare themselves very quickly and you’ll be able to decide then if you need that break to bring the dose down. I would say 12 mg has been the standard, and I would not make shortcuts on this because we might not necessarily benefit the patient. Moreover, I would say the combination of atezolizumab and bevacizumab, in that case, is probably not the right answer.
Lenvatinib or sorafenib are appropriate. Oxaliplatin, fluorouracil, and folinic acid [FOLFOX], interestingly, is a standard of care, but not in the United States, whereas in China it’s an appropriate first-line therapy.4 Nivolumab [Opdivo] was also pulled out of the market for that purpose.5 It did not benefit patients because the study was negative, and I would not bring the nivolumab as a standard of care.The guidelines from the National Comprehensive Cancer Network [NCCN] classify atezolizumab/bevacizumab as A1, sorafenib as A1 or B7, and lenvatinib as A1.
Interestingly, they are still kind of chatting about nivolumab because they have it for Child-Pugh B, but I don’t know, the NCCN is going to probably revise it because the drug is not yet approved and the request for approval was pulled out.6 FOLFOX was kind of determined as an option simply because of the data that came out for the FOLFOX regimen when they compared it with doxorubicin a long time ago.4
The question [when looking at these various options is]: How can we get a little bit of a better read on this? Of course, more data, better data, improved outcomes are needed, but that’s the beauty of us talking to each other [at live events like the Case-Based Roundtable Series], that we learn from each other what could be the important variables that can decide one way or the other.
Systemic Therapy vs Interventional Radiology Therapy
Historically, the interventional radiology people have been heavily involved because, humbly and sadly, we did not have options. But now we are loaded with options, and if anything, patients know about the options, and they want to get a [better treatment] opportunity from them.
So the best data we have come from Japan where if the disease is limited to the liver, because remember if it’s metastatic then it’s systemic therapy for sure, but if it’s limited to the liver and the number of lesions is counted [patients may be better candidates for systemic therapy].7
Let’s say, for argument’s sake, there are 4 lesions in the liver and if you add the size of the largest lesion, which, let’s say, is 4 cm, is equal to 8 [lesions]. [The researchers from Japan then] found out that patients who have more than 7 points, what they call up to 7 criteria based on transplant data, patients will not do very well on the local therapy. Therefore, it would be recommended to do systemic therapy.
In their trial, they randomized patients with up to 7 criteria with only locally advanced disease in the liver, and they randomized them to embolizations, same way that one would see patients do vs lenvatinib.
The median survival for the embolization was at 20 months and that’s exactly what has been reported by a couple of groups—interestingly, for the patients on lenvatinib, for local therapy for 58 months, which I think tells us something. So I would say [to other clinicians in this situation to] please make sure if you have a tumor board, and if you have access to your tumor board, to always be there to make sure you are heard. The data are there, and one can definitely stand in a meeting and say [that if there are more than 7 criteria determined like on this study], then I think it’s time to use systemic therapy.
Data Backing the Choice of Lenvatinib
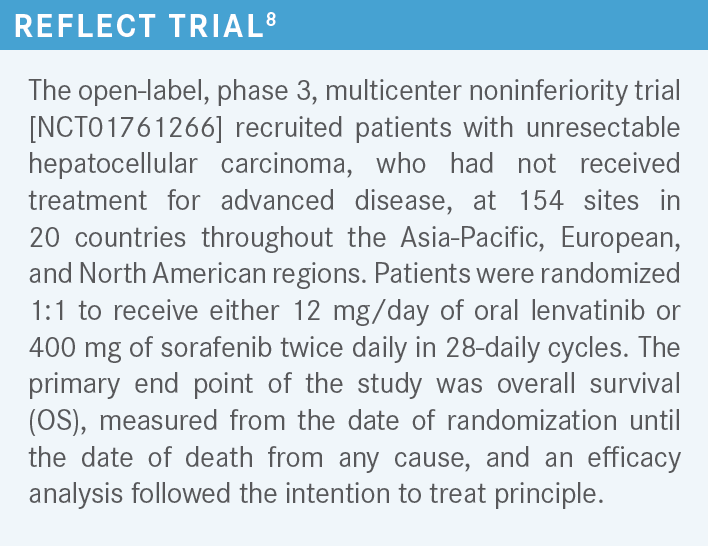
[The first complex results comparing these 2 drugs came] from the REFLECT trial.8 The study was positive because it looked at noninferiority and there was no inferiority observed. Interestingly, it showed a new median OS of 13.6 months for patients on lenvatinib compared with 12.3 months for patients on sorafenib, with a hazard ratio of 0.92 (95% CI, 0.79-1.06).
Now, we know that lenvatinib has an anti-EGFR activity that is very robust, and that activity is about 80 times higher than sorafenib. That’s why we use only 12 mg of lenvatinib while we use 800 mg of sorafenib. Interestingly, this reflected on the progression-free survival [PFS], which was almost close to 3 times higher for patients on lenvatinib at [a median of 7.4 months vs 3.7 months (HR 0.66; 95% CI, 0.57-0.77; P < .0001)]. So [if you think about it like] you have a racetrack, which is exactly 1 length, it does not mean you cannot have a faster car, and that’s where the faster car of the lenvatinib comes into play for this disease.
Second-Line Therapy After Disease Progression
I personally stand by still using lenvatinib [in this setting] because we’re not going to discredit the first-line therapy, like lenvatinib, sorafenib, and of course, if anybody progresses on atezolizumab plus bevacizumab, it’s still not a bad idea to get them to the lenvatinib as second line.
When looking again at the NCCN guidelines, it’s amazing that they said subsequent-line therapy in this disease progression does include lenvatinib and sorafenib. Interestingly, I notice that there’s a lot of complexity with too many drugs coming very quickly, enough that it might not even catch up with first and second line.
I understand that people might be concerned about the safety and tolerability [of the drug] in the second line, but I could assure [people with those concerns that] in the old days, maybe 15 years ago, if you asked me about the second line, I’d tell you I’m not sure that patients will do well. We have also come out with great therapies these days and people do very well. So don’t discredit that the patient might do very well in that regard. If anything, in the second line, the options for therapy include lenvatinib, cabozantinib, and sorafenib, and I know many that would choose lenvatinib, which is my default [in this setting] as well.
REFERENCES
1. Kumar A, Le DT. Hepatocellular carcinoma regression after cessation of immunosuppressive therapy. J Clin Oncol. 2016;34(10):e90-e92. doi:10.1200/ JCO.2013.51.4067
2. Chen J, Tseng Y, Luo T, Li N, Ma L, Chen S. Prophylactic endoscopic therapy for variceal bleeding in patients with hepatocellular carcinoma. J Cancer. 2019;10(14):3087-3093. doi:10.7150/jca.30434
3. Lenvima. Prescribing information. FDA. Accessed June 22, 2022. https://bit. ly/3HT3U8r
4. Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014;19(11):1169-1178. doi:10.1634/theoncologist.2014-0190
5. Bristol Myers Squibb statement on Opdivo (nivolumab) monotherapy post-sorafenib hepatocellular carcinoma U.S. indication. News release. Bristol Myers Squibb. July 23, 2021. Accessed June 15, 2022. https://bit.ly/2UOBmZI
6. NCCN. Clinical Practice Guidelines in Oncology. Hepatobiliary cancers, version 4.2021. Accessed September 17, 2021. https://bit.ly/3Ao3MZW
7. Kudo M. Surveillance, diagnosis, and treatment outcomes of hepatocellular carcinoma in Japan: 2021 update. Liver Cancer. 2021;10(3):167-180. doi:10.1159/000516491
8. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi:10.1016/ S0140-6736(18)30207-1

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More