Roundtable Discussion: Jonasch Discusses the Management of Clear Cell RCC
During a Targeted Oncology case-based roundtable event in Texas, Eric Jonasch, MD, moderated a discussion around the management of clear cell renal cell carcinoma in a 59-year-old African American female patient.
Eric Jonasch, MD
Professor, Genitourinary Medical Oncology
Graduate School of Biomedical Sciences
The University of Texas MD Anderson Medical Center
Houston, TX

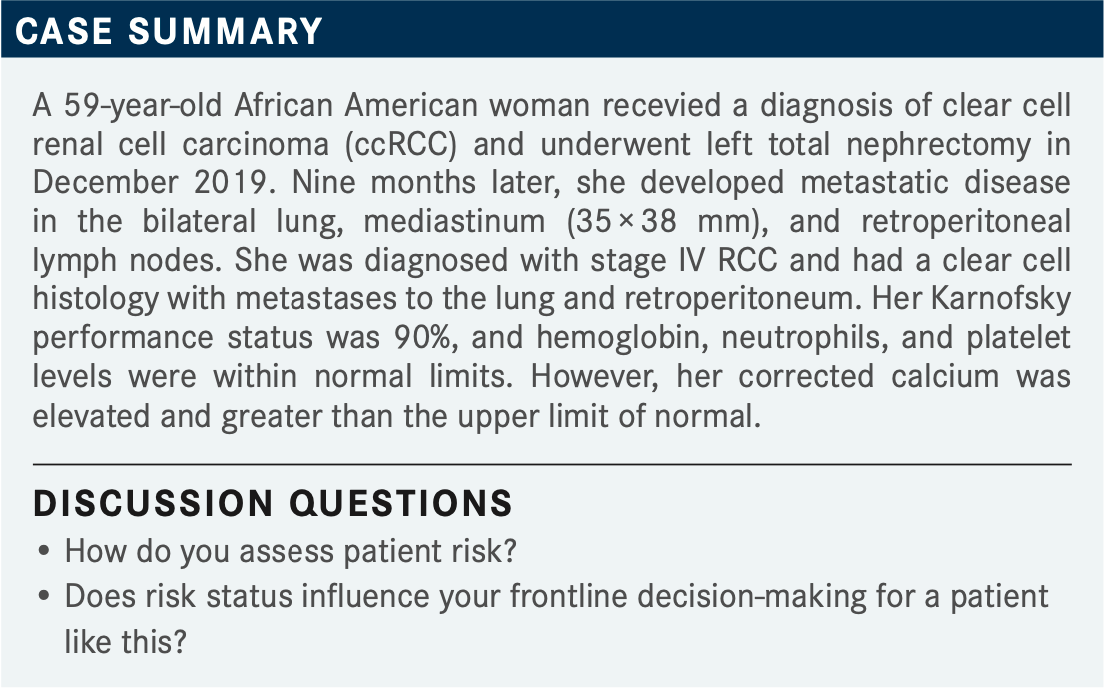
JONASCH: This is a woman who is presenting 9 months after a nephrectomy with metastatic disease, [with] most laboratory parameters normal except for the elevation of calcium. What are the algorithms you use to define a patient’s risk? What is your approach in this?
LUU: I break it down to low, intermediate, or high risk, based on the International Metastatic RCC Database Consortium [IMDC] risk calculator. Then, if [a patient] has low risk, I will rule out ipilimumab [Yervoy] plus nivolumab [Opdivo].
JONASCH: The IMDC risk calculator is very similar in composition [to the Memorial Sloan Kettering Cancer Center risk assessment calculator], but both provide very valuable information. Most of the newer studies classify patients using the IMDC risk algorithm, so it is the most current one.
Does risk status influence your frontline decision-making? It sounds like Dr Luu is going to be ruling out certain therapies if the patient is at favorable risk.
REDDY: The frontline regimens [that are recommended by] the National Comprehensive Cancer Network keep changing [from what they were] 2 years ago, 12 months ago, [and even] 6 months ago. I do not see a new metastatic patient every month, so I am not [always] that current. In the most recent [guidelines], we have the cabozantinib [Cabometyx] and nivolumab [combination recommended] for all risk categories.1
I feel like [risk category] does not matter. We could use the cabozantinib plus nivolumab combination for all risk groups.
SHIMKUS: We [take risk status into consideration], but [most of] my patients tend to be older, and most of them end up being high risk, so I check for completeness’ sake. I agree, the regimens keep changing, and occasionally we get into problems with some insurers about how [one should] use first-line and second-line therapies, [about] when [one] can use the dual immunotherapies [IOs], and how that impacts treatment choices down the road. But we do use [risk status]—it’s in every chart, but again, most people end up being high risk or at least intermediate risk.
AMBIKA: Were brain metastases or liver metastases [taken into consideration when the risk model was developed]? Because, biologically, you feel like they are more aggressive, right?
JONASCH: It was a multivariable risk model that was developed, using several different variables. Whether they looked at brain metastasis as an individual variable, and whether that ended up being statistically positive in their model, I don’t know. One of the interesting challenges here is to have enough individuals who have a particular feature to be able to put it into a multivariable model. You are right, though—liver metastases, brain metastases—these are features that are associated with much worse outcomes, and we do take these things into consideration when we look at our patients. Other things, [such as] bone metastases, could be put into [the calculation], as well. But basically, [the risk calculation uses the factors that were] positive in the multivariable model.

AMBIKA: We tend to do a PET scan or a bone scan, and a brain MRI for sure, then start treatment.
MOSCOL: I usually use CT scans [rather than PET scans]. I think it is more difficult to justify using the PET [scan], and I get a little more resistance from the insurance companies. I tend to do bone scans whenever I am monitoring.
JONASCH: The brain MRI and potentially the bone scan [are] some things we would want to look at, because there is a small percentage of individuals who do have de novo brain metastases and, sometimes, bone metastases in the appendicular skeleton. You don’t see it on the CT scan.
I also agree that a contrast CT would be the standard of care for an individual with metastatic RCC [renal cell carcinoma]. PET can be very valuable in RCC—in an individual where PET is positive. The problem is that sometimes the RCC tumors are not high-glucose users, and you will end up having a negative PET [scan result, which] creates a lot of confusion.
If the patient’s glomerular filtration rate is reasonable, I tend to use the CT scan with contrast. Now for this patient, would you initiate systemic therapy at this point, or would you watch and wait?
MANGAT: With intermediate risk, I would lean toward treating this patient.
SHIMKUS: [There has been] too short of a time from diagnosis to presentation with metastatic disease to justify observation.
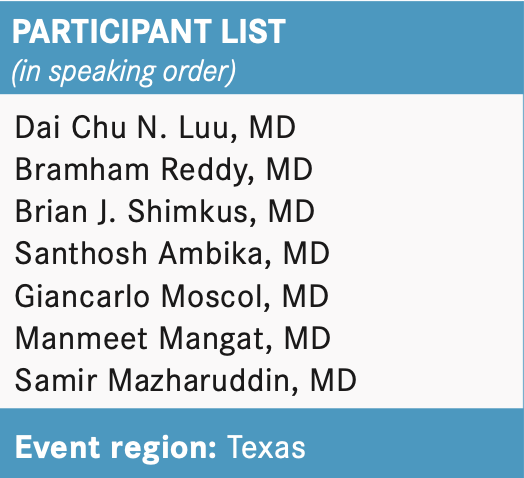
AMBIKA: Yes, I have 2 patients [on lenvatinib who have been doing OK]. One patient has done very well; she is almost 3 months [into] the treatment. The other patient was just started recently.
However, I had a question about the CLEAR [NCT02811861] trial. I saw the quality-of-life data, and they said [the experimental regimen was] better than sunitinib, which was a low threshold [to clear]. In general, lenvatinib is not an easy drug to handle, so it does not make sense that it [offers a] better quality of life.
JONASCH: Yes, we can talk about that. Quality-of-life tools are [ambiguous]. [It is hard to say] what it exactly means when you see separation of lines on a figure.
MAZHARUDDIN: I have used [this regimen] in 2 patients. Both responded, which is nice. One of them is just sailing through the lenvatinib—he is doing well. He has adverse effects, but he is very motivated and is tolerating the full 20 mg. The other [patient] is feeling quite weak and having complaints of stomach pains and things like that. Nevertheless, [this patient is] doing relatively well on it.
JONASCH: [The combination of lenvatinib plus pembrolizumab] is relatively early in its life span, if you will. It was only FDA approved in August 2021.2 What are your reactions to these data?
SHIMKUS: It was interesting to see how well lenvatinib seemed to do, but I was also a bit surprised by how poor the data were for ipilimumab plus nivolumab [CheckMate 214; NCT02231749]. I consider that to be a more aggressive [regimen]. I use that for my patients [because] I need a durable response. Maybe that is [misguided], but my perception [has been] that the dual IO [combination] is the more aggressive regimen. Maybe it is time to reconsider that.
JONASCH: Right, I share your view on that. There is no question, though, that ipilimumab plus nivolumab [has something to] offer: a complete response [CR] rate that is greater than 10%, and that CR can be durable.3 Also, individuals who are on that regimen, once they get through [treatment with the combination], can have a nice life on single-agent nivolumab. The real challenge with it is that you have such a high [rate of] progressive disease as best response,4 relative to the other regimen.5 There is a balancing act here.
REDDY: The first question is whether to use [the combination of] ipilimumab plus nivolumab vs a tyrosine kinase inhibitor [TKI] plus [a drug that targets the PD-1 pathway]. The next question [is how to choose from among] the TKI and PD-1 drugs. I would think they are all similar, but if I made up my mind to use a TKI and a PD-1–targeting drug, how would one decide? Do we have anything to say one combination is better than [another]?
JONASCH: I do not think we can say that. I think we need to look at the numbers. There are clearly numerical differences. The CLEAR data are numerically the nicest— an objective response rate [ORR] of 71%, a CR rate of 16%, and [a median] progression-free survival [PFS] of 23.9 months.5 But in the CheckMate 9ER trial [NCT03141177], [the combination of cabozantinib plus nivolumab] had a CR rate of close to 10%, a PFS of nearly 17 months, and an OS advantage.6
REDDY: We tend to think most of [the] PD-1 drugs are the same, but is there any reason to think [any] of the TKIs is better than another? If so, which TKI would you think is the [best, from among axitinib (Inlyta), cabozantinib, and lenvatinib]?
JONASCH: I think they are all good. The first question we need to ask ourselves, [as was mentioned earlier, is whether to choose an] IO plus TKI regimen [or] an IO plus IO regimen.
REDDY: In that sense, the decision is simple. But once you decide [to use, for example], an IO plus TKI combination—[which is] what I was thinking—we do not have a direct comparison of any of these combinations [to assist us in choosing from among axitinib plus pembrolizumab, cabozantinib plus nivolumab, or lenvatinib plus pembrolizumab]. The next [best] way I can [choose is to consider which] TKI is considered superior.
JONASCH: As we saw with the data, there are differences. The ORRs and [other measures of efficacy] are higher with lenvatinib plus pembrolizumab, [as were] the toxicities,5 so there is a balancing act. Perhaps in a patient where you are more concerned about the TKI toxicities, maybe the combinations of cabozantinib plus nivolumab or axitinib plus pembrolizumab might be better. Having said that, you could dose reduce, and you could start at 20 mg of lenvatinib. That, perhaps, makes [the regimens] very similar. Your point is excellent; we do not have cross-trial comparisons here, and we have similar regimens with numerical differences. What some of my colleagues say in this situation is to pick an IO plus TKI regimen and get good at that specific regimen. Learn it, learn how to manage it, and learn how to deal with the logistics of dose reduction. Then you have your ipilimumab plus nivolumab for the [patients who require] it.
MOSCOL: Do you know [how the] duration of response differs for lenvatinib plus pembrolizumab vs ipilimumab plus nivolumab? Are the data mature yet?
JONASCH: The duration of response for the lenvatinib plus pembrolizumab regimen was [approximately] 17 months.5 For ipilimumab plus nivolumab, what I can tell you is that for individuals who have a response at 2 years, the probability of maintaining that response exceeds 80%.3 The data for the lenvatinib plus pembrolizumab combination are not quite mature, but for people who do respond to ipilimumab plus nivolumab, the probability of maintaining that response is quite high.
MANGAT: Does the CLEAR data change your mind [about] what [treatment] to choose?
JONASCH: For ipilimumab plus nivolumab, you are going to have a 60% ORR—incredible data, durable response. But the combinations of axitinib plus pembrolizumab [KEYNOTE-426; NCT02853331], cabozantinib plus nivolumab, and lenvatinib plus pembrolizumab7 all show clearly favorable ORRs and PFS compared with sunitinib. I think all of them are good. If you have a patient who has nonthreatening disease, but with sarcomatoid features, ipilimumab plus nivolumab is a fantastic choice.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2022. Accessed March 26, 2022. https://bit.ly/36sAkse
2. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. Updated August 11, 2021. Accessed March 28, 2022. https://bit.ly/3rzXK6f
3. Motzer RJ, Tannir NM, McDermott DF, et al. Conditional survival and 5-year follow-up in CheckMate 214: first-line nivolumab + ipilimumab (N+I) versus suni- tinib (S) in advanced renal cell carcinoma (aRCC). Ann Oncol. 2021;32(suppl 5):S685-S687. doi:10.1016/j.annonc.2021.08.057
4. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi:10.1136/esmoopen-2020-001079
5. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
6. Choueiri TK, Powles T, Burotto M, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31(suppl 4):S1159. doi:10.1016/j.annonc.2020.08.2257
7. Choueiri TK, Eto M, Kopyltsov E, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Ann Oncol. 2021;32(suppl 5):S683-S685. doi:10.1016/j.annonc.2021.08.056

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen