Roundtable Discussion: Saba Reviews Testing, Therapies, and Toxicity With Participants
Nakhle Saba, MD, reviewed testing, treatment, and treatment toxicity in a 71-year-old woman with chronic lymphocytic leukemia with a group of peers during a Targeted Oncology case-based roundtable event.
Nakhle Saba, MD


SABA: Why do you think we are seeing that most people are not testing? Is it because of the cost, availability? It doesn’t matter anymore? Is it one- size-fits-all treatment—you just start the treatment, and it’s going to work regardless?
ZOGHBI: Yes, I think it is several factors taken together. One of the problems I noticed is sometimes when I submit those tests, insurance doesn’t want to pay for them. I have had to apply and say these are for prognostic [purposes].
But [for patients who are] asymptomatic, newly diagnosed, sometimes insurances just don’t want to pay for it. I don’t know why they call it under genetic or [if there’s] some stuff they just don’t want to pay for. I think that is one. I have seen it in my practice. Not all the patients, but quite a [number] of patients.
I think the question becomes: Do we need it, or do we already have our best drug up front? Do we choose ibrutinib [Imbruvica] or acalabrutinib [Calquence] vs venetoclax [Venclexta] or obinutuzumab [Gazyva], and it doesn’t matter anymore? I still like to get testing done because [it gives] us prognostic information. Now I don’t know in the long term...how much those drugs with the unfavorable prognoses [may] change, or if it matters, but this could also be a reason. I think availability might be an issue as well, especially in smaller practices or practices not affiliated with any university.
SABA: Remember everyone, you don’t have to do a bone marrow biopsy for this. Whatever you have access to, tumor cells or cell, this is where you can take those cells and test them. Blood is perfectly fine. IGHV does not change [with] disease progression—first line, second line, it doesn’t matter. IGHV mutation is the same mutation [once] it has mutated. The same V gene is not going to change throughout the natural history of your patients’ disease, as opposed to FISH. That has changed over time at the rate of about 15% over 5 years or so. Similarly, the 17p clone that was not there might appear at progression down the road, etc. So based on what you are saying, Dr Zoghbi, you don’t think this pattern will change, or is changing? I don’t think I am seeing it changing—that people might not necessarily be interested in knowing or testing in the future for the reason you stated. How do you see the future for this? Do you think it might improve?
ZOGHBI: I think that all the time on the data, I see more and more coming, and [they give] us information. The information has been there and has changed over time when a new is drug coming....I suppose when a patient progresses, [you want to look to see that] something else hasn’t changed. I was a little bit surprised that on relapsed or refractory, there are, in fact, more people not testing. When patients progress, [I feel like you would] try to look up, what’s my next choice? What’s my next option?
We know physicians in other diseases now are doing it even more. The patient progresses, [so] maybe we check another mutation and analysis to see if anything comes up for availability so we have another line of therapy.
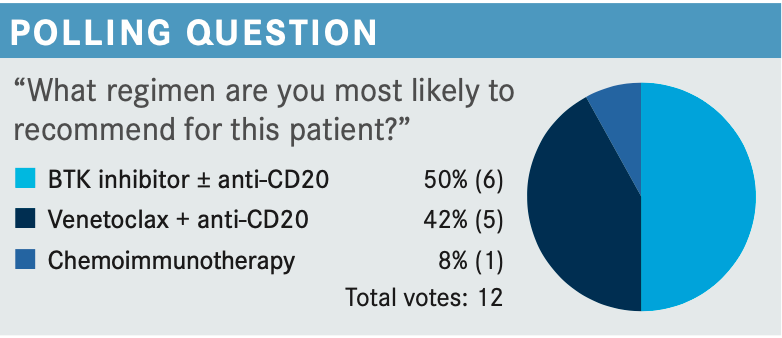
SABA: BTK [Bruton tyrosine kinase] inhibition. Is it one size fits all? Is it BTK inhibitors for everyone? Or is there something particular about this patient that gets you to choose a BTK inhibitor? Or would you also use venetoclax plus obinutuzumab?
RAJA: I chose the BTK inhibitor, that is my go-to option for frontline therapy. This patient also has del11q. I know it’s not del17p, but [it’s] still high-risk disease. So I still think a BTK inhibitor would be a good option. I have not started using venetoclax plus anti-CD20 for first-line therapy yet, but that is on my radar. [I’m] still a little hesitant about using venetoclax in outpatient therapy with tumor lysis syndrome and all that. But, yes, that is something that I may start adopting in the future, just because of its finite duration, which can be an advantage over the BTK inhibitor.
About the BTK inhibitor, only recently I have started using more of them, ibrutinib and acalabrutinib, because of the slightly better toxicity profile. This patient is on an acid reducer, and I think acalabrutinib may become a little bit problematic for this patient because of its potential interaction. So I would probably pick ibrutinib as a single agent for this patient.
SABA: Right now, BTK inhibitor is your go-to front line unless there is something that prevents you from using it. Can you share any reason that you might say you’re not going to use a BTK inhibitor, ibrutinib or acalabrutinib, in this patient?
RAJA: If somebody starts a finite therapy, maybe I would pick something else. Venetoclax and anti-CD20 would be a good option, especially in this high-risk disease with deletion 11q. I am not comfortable using chemoimmunotherapy in this situation, again because of del11q.
SABA: For other users of BTK inhibitor, does anyone use an anti-CD20 with it?
SUMOZA: One thing we have to keep in mind is that in the ELEVATE CLL TN trial [NCT02475681], which is acalabrutinib plus obinutuzumab, most of the patients who benefit are the ones who have immunoglobulin inhibition–mutated [disease], and these patients are unmutated. So that was the reason for me to choose venetoclax over anti-CD20.
SABA: [This is all covered by] the ELEVATE study [NCT01722487], the RESONATE-2 study [NCT01724346], and the CLL14 study [NCT02242942] on the front line. Is anyone using an anti-CD20 with a BTK inhibitor, whether ibrutinib or acalabrutinib? I am guessing this is a no. Chemoimmunotherapy? I know [it is] still under the NCCN [National Comprehensive Cancer Network] recommendations, but how do you feel about chemoimmunotherapy?1
MATTAR: I have not done that in the last 4 or 5 years. Recently I have not seen any indication to do chemotherapy. And I know NCCN put it in the recommendations, but in the real world when you can do a BTK inhibitor or venetoclax, I don’t know.1 I have not done that, honestly, for 3 or 4 years. Now with the coming of chimeric antigen receptor [CAR] T and [treatments] like this down the road, even for third and fourth line, I am not inclined to use chemotherapy anymore, other than as part of CAR T.
HUANG: Chemoimmunotherapy may be an option for patients who want to have a limited treatment instead of continuing treatment with the BTK inhibitor. It’s not for everybody.
GUPTA: Even for limited-time therapy, I would rather go with a venetoclax-based regimen than chemoimmunotherapy.
MATTAR: I chose venetoclax for a limited option. For my first line, I have chosen venetoclax because I want finite therapy. I don’t want it to go forever. Six cycles of obinutuzumab plus venetoclax after 1 to 2 years, and then that’s it.
SABA: Venetoclax-based therapy offers this fixed-duration treatment if you don’t want to go for a lifelong commitment in this case.
SUMOZA: Chemoimmunotherapy is for younger patients, especially if they have the immunoglobulin-inhibiting mutation, and this is not the patient for that.
SABA: So you still consider it in CLL possibly [for those who are] young, fit, IGHV mutated, with no del(11q), no TP53 alteration because you can get a long-term remission/cure in about 50% of patients. But remember, those are the ones who are going to achieve minimal residual disease [MRD] negativity at the end of fludarabine, cyclophosphamide, rituximab [Rituxan] [FCR] treatment. You don’t know until after you finish up FCR, and that is problematic. Then you have the problem of myeloid malignancies down the road to also keep in mind. And if you don’t get MRD negativity, [that’s an issue]. But that is definitely, in my opinion, the only place where you possibly can argue a role for chemoimmunotherapy in CLL.

SABA: We are talking hypertension, atrial fibrillation [Afib], flutter, the need of anticoagulation in the setting of Afib. Do you, for example, use the help of a specialist such as a cardiologist, particularly a cardio-oncologist? Do you have a cardio-oncologist? If you don’t have one, did you make one in your practice like I did? I sent all my patients to just 1 cardiologist, and he became my cardio-oncologist.
MATTAR: I made our cardiologist switch after talking to him about the CAR T cells. I made him the CAR T cardiologist, giving him any article about that. He became one of our doctors because he showed interest. He is not an interventionist.
OPPELT: I am lucky to have not 1 but 2 cardio-oncologists who are dedicated to the practice, so we utilize them frequently.
SABA: OK, great. If your patient is on a BTK inhibitor and developed Afib, do you take them off therapy regardless, or does the grade of Afib matter? Some people say it doesn’t matter what grade it is. Some people say that grade 1 and 2 could be symptomatic and that I might detect it if I checked the pulse of the patient—and that a BTK inhibitor might not necessarily make it worse, especially if I treat it. How do you approach Afib?
SUMOZA: One other thing is, though, it is not only the Afib. It is that the patient has to be on blood thinners, and then you may have their risk factor for bleeding.
SABA: So Dr Sumoza, is Afib is a deal breaker? [Would you] just stop the BTK inhibitor altogether and move on to next line? Or can you still manage some patients with anticoagulation, if needed, and Afib therapy, knowing that there is no contraindication for both drugs?
SUMOZA: I send the patients to the cardiologist. But I have a patient who developed Afib. He was on a blood thinner. But he was very comfortable with ibrutinib, and he had a good response. So for this patient, we reduced the dose of the ibrutinib, and he was still on the blood thinner, but the Afib was very bad. And then, after reducing the dose, we finally stopped it and moved on to another regimen.
MATTAR: Does all Afib have to be from the BTK inhibitor? Could it not be just de novo Afib like any older person can have, and it happened to be that he is on a BTK inhibitor? That’s the way I think about it.
SABA: Definitely. If you have a patient with Afib, do you feel comfortable starting, let’s say, a BTK inhibitor?
GALAMAGA: I’d feel comfortable, but maybe with something like acalabrutinib rather than ibrutinib. I have moved away from using ibrutinib because of the data in regard to Afib and bleeding. I have a comfort level [with acalabrutinib]. If we lay all the options out on the table and have an informed discussion with the patient, then I have a comfort level. But mostly with the second generation and beyond.
SABA: That is based on the ELEVATE [CLL] TN relapsed/ refractory [data].2 But assuming someone has Afib, have you seen or experienced people get worse? They already have Afib, or does it matter? Once they have it, BTK inhibitors might not necessarily make it worse. Do you still use that acalabrutinib and dexamethasone?
GALAMAGA: I have not seen it get worse, though. I don’t know of data [showing] it getting worse on the second generation.
SABA: Me either. But, again, there’s the issue with the blood thinners and the bleeding, for example. Again, there is no contraindication. You can use it, but you have to be safe, and you have to weigh the risks and benefits.
Let’s talk about adherence. Does it matter [if you are giving it] once a day or twice a day?
BANDI: I guess, if the prescription is supposed to be given every 12 hours, I would be concerned if they don’t take it. Once a day is much better than twice a day for many patients. It’s hard to remember, and some purposely try to ignore it because of adverse events. So I would prefer, if possible, to do once a day rather than twice a day, but sometimes we don’t have a choice. And if I do that, I will stress the importance of sticking to the regimen twice a day if we want to see the desired effects. I have seen people being noncompliant—not by choice. It just happens.
SABA: Another thing I mention when I compare the label and the interaction with other drugs is the acid reducers and PPIs [proton-pump inhibitors].3 Dr Baldeo, what do you think about this? Are you aware of the H2 [histamine 2] blockers, for example, and the PPI that you cannot combine? Do you counsel your patients about that? Sometimes we have a lot of patients who take that.
BALDEO: Yes, sometimes we don’t even know these patients are taking it. They take it over the counter, and it is not mentioned in their medication list. So I would warn them about it, especially if they are on acalabrutinib, but because of the once-a-day ibrutinib we lean toward the ibrutinib.
SABA: So you give your patients the options, you explain the differences and pros and cons of each, and you let them weigh in to this. But you are also bringing up an important point that a lot of patients don’t even mention their PPI because it’s an over the counter. I always ask myself about this, particularly if they are on acalabrutinib.
SABA: Is there anything that makes you choose [one or the other], particularly about the risk factors in CLL. Is there anything that makes you chose lifelong, continuous suppression vs fixed duration? Particularly here I am talking del(17p). If you have del(17p) and a TP53 mutation, do you feel comfortable stopping at 1 year with venetoclax plus obinutuzumab, or do you move to a BTK inhibitor?

FIRWANA: Usually venetoclax plus obinutuzumab is my first line in most patients, even in [older] patients. I notice that venetoclax is very well tolerated. I have used it in different settings in CLL as well as in high-risk myelodysplastic syndrome and acute myeloid leukemia. It is very well tolerated. Using it for a year, it is a big advantage, but also, the tolerance offered makes it more appealing to discuss it with patients.
SABA: Regardless of the risk factors, you still feel comfortable, even in the setting of del(17p)?
FIRWANA: I do. I think we are not burning bridges if we use it and then keep a close eye and monitor, and then we still have the other options to use in case there is a progression. We had a trial at our center [with] all 3 treatments: venetoclax, BTK inhibitor, and rituximab. This can become an option in high-risk patients if it showed better outcomes, better responses down the road.
SABA: Yes, you are right. There [are] no data to say that if you do venetoclax plus obinutuzumab and stop in those with del(17p), those patients are going to do worse if you treat with a BTK inhibitor down the road. But it seems to be a common practice across a lot of people to do continuous suppression in that setting.
ZOGHBI: I agree. A lot of patients I present both [options] to...tend to choose the limited time. I feel they would reserve the long-term treatment for the next-line option. So I do offer [BTK inhibitors] even for those with del(17p).
To answer the question about do we use acalabrutinib twice a day or once a day, I have a patient who developed a severe headache and had significant toxicity with acalabrutinib, so we decided to reduce the dose. I tried to treat it and give her more caffeine.
I did start once a day [dosing], and I feel over 1 or 2 weeks, her symptoms improved. It was under control. So I was able to go back to the twice a day. But talking to the pharmacist, I think pharmacodynamically, to have benefit, you need to keep the twice-a-day dose.
SABA: What about [COVID-19] with anti-CD20? Did that make you change your practice at all? A lot of people moved to a BTK inhibitor, stayed away from anti-CD20 not to deplete the B cell with regard to anti–[COVID-19] defense. Did it affect any of your practices? Is it changing now with things getting better with vaccination and more treatment available?
MATTAR: It made me anxious earlier on, but now it is better and better. I started giving every patient with lymphoma the tixagevimab plus cilgavimab [Evusheld]. I am pulling them from home to come and get the tixagevimab plus cilgavimab after their [COVID-19] vaccination.
The other question is about the 1 year. I need your input, Dr Saba. Would [I] check if I [have a patient with] del(17p)? I don’t know. I don’t have enough data, but del(17p) after 1 year, would you check about the MRD in that setting because I know it is still controversial? If I get MRD, then I feel more comfortable to start vs just having the normal white blood cell count.
SABA: I think the future will let us know whether the MRD will have to continue or not. But based on the CLL14 trial, they did check MRD—and MRD negativity at the end of treatment did much better than MRD positive.4 We know that. I check it just for prognostication, but I don’t base my full therapy on that. So if I am selling my patient on a therapy of fixed duration, I’m doing it. I’m going cold turkey. I am stopping at the 1 year. I check MRD to just see how they are going to do in the future. But I just stop and monitor for these progressions.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Chronic lymphocytic leukemia/ small lymphocytic lymphoma, version 2.2022. Accessed April 1, 2022. https:// bit.ly/37BuHby
2. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutu- zumab versus chlorambucil and obinutuzumab for treatment-naive chronic lympho- cytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
3. Calquence. Prescribing information. AstraZeneca; 2022. Accessed April 1, 2022. https://bit.ly/3EVLi6a
4. Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lympho- cytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188-1200. doi:10.1016/ S1470-2045(20)30443-5
