Roundtable Discussion: Budde Focuses on the Factors Affecting Treatment Decisions in B-cell Lymphoma
During a Targeted Oncology case-based event, Elizabeth Budde, MD, PhD, discussed with participants the case of a patient with relapsed/refractory diffuse large B-cell lymphoma who was not eligible for stem cell transplant and declined chimeric antigen receptor T-cell therapy.

Elizabeth Budde, MD, PhD (Moderator)
Associate Professor, Division of Lymphoma
Department of Hematology & Hematopoietic Cell Transplantation
City of Hope
Duarte, CA


BUDDE: Some factors to consider include IPI score at diagnosis, cell of origin, prior therapy received, eligibility for allotransplant, eligibility for chimeric antigen receptor [CAR] T-cell therapy, efficacy of the regimen, safety and tolerability of the regimen, the patient’s comorbidities and performance status, and patient preference. Do we also consider convenience or logistics? What about insurance coverage? Are there other factors to consider?
SWEET: Usually my first call after I have somebody with relapsed disease is to the transplant team I work with, and they’ll be the ones to determine eligibility for either transplant or CAR T-cell therapy. In a way, as a frontline oncologist, I’m deferring to those specialists to gauge the appropriateness of [preparing] that patient for a transplant or CAR T-cell therapy. Absent that, if the patient is not eligible, we [would consider] GemOx [gemcitabine, hydrochloride, and oxaliplatin] or polatuzumab vedotin-piiq [Polivy], predominantly. Again, [we would do this] somewhat in concert with [the transplant team’s] recommendations, [because] for most of us, as general oncologists, we see only a few of these patients a year. The [transplant team] sees a very enriched population, and I can benefit from their expertise.
BUDDE: You’re alluding to a good collaboration with academically informed specialists. You probably know the patient better, and they probably know the literature better, so a good collaboration between the 2 can come up with the best regimen for the patient.
LORBER: I agree [with Dr Sweet]. Generally speaking, if it’s relapsed disease, the first thing we go to is autotransplant. After failure of that, usually, if the patient still has a decent performance status, I go with CAR T-cell therapy. I’ll refer the patient to CAR T-cell therapy if we’re unable to find a spot for them at our institution.
CHAND: It is common practice to refer out to transplant attendings at tertiary care [centers] for consultation if the patient had a complete response after the use of R-CHOP [rituximab (Rituxan), cyclophosphamide (Cytoxan), doxorubicin, vincristine, and prednisone], but there is a small class of patients with DLBCL who don’t have a CR [complete response]. Is [such a] patient ever going to be a transplant candidate? I want to hear your opinion about sending a patient for a transplant consultation if the patient isn’t chemotherapy sensitive and didn’t have a CR to begin with.
BUDDE: We know that for patients with primary-refractory disease, if they never achieved a good remission to the first-line chemotherapy-based regimens, their response to a second-line regimen may not be as good as [that of] someone who has no recurrent disease. However, the second line of therapy is going to be a different chemotherapy regimen, so there are patients who respond to the second-line therapy and achieve CR. Autologous stem cell transplant is still the subsequent line for consolidation, and if a patient has a CR to second-line chemotherapy, they are considered to have chemotherapy-sensitive disease.
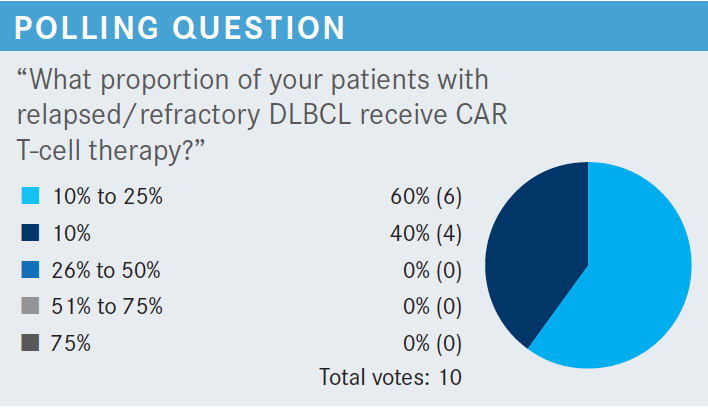
HSU: In [most] cases, the [patients are] sick and their cancer progressed at a rapid rate, and the criteria for CAR T-cell therapy are pretty stringent in the facility we’re affiliated with, so the patients are never well enough [to receive CAR T-cell therapy]. I feel the main reason [for patients not to receive CAR T-cell therapy] is that the disease progressed and could not be controlled. Also, some people are too well for CAR T-cell therapy.
BUDDE: For this patient, CAR T-cell therapy was more of a logistical concern, but these 3 regimens are pretty good options. When you’re choosing from among them, does future potential for CAR T-cell therapy matter for you? Let’s say you have a patient, similar to the one we are discussing, who has now had 2 prior lines of therapy, R-CHOP and GemOx, and now we are coming to pick a third-line regimen. For this patient, which of these regimens would you pick?
LORBER: If the plan were for the patient to get CAR T-cell therapy in the future, then I would choose the combination of polatuzumab, bendamustine, and rituximab, because it doesn’t have any type of reactivity. You’re not targeting the same antigen that tafasitamab or loncastuximab target. If CAR T-cell therapy were not an option, I would choose one of the other 2 regimens [tafasitamab plus lenalidomide or loncastuximab tesirine] if the patient were refractory to both R-CHOP and R-GemOx. With the polatuzumab, bendamustine, and rituximab combination, polatuzumab is a novel CD79B-targeting agent, but bendamustine is still typical alkylator chemotherapy and rituximab is still rituximab. I would add the caveat that, for a patient who is a candidate for CAR T-cell therapy but who has refractory disease, I would probably choose lenalidomide plus rituximab, because at least with the lenalidomide, you might be able to control the disease a little better than [if you were to] just hit the patient with more traditional chemotherapy.
CHAND: [How long after] bendamustine exposure [must a patient wait to safely receive] CAR T-cell therapy?
BUDDE: [That is to say] if we give a patient polatuzumab, bendamustine, and rituximab, will this affect the patient’s T-cell quality and their ability to have a good response to subsequent CAR T-cell therapy? We don’t know how many months you need to be free of bendamustine exposure to have good T cells, but I know there is a study coming out. A group at [The University of Texas] MD Anderson Cancer Center has put together a retrospective review looking at the outcomes of patients with bendamustine exposure and correlating [the time since exposure] at 6 months, a year, or 2 years, with response to CAR T-cell therapy. In my own practice, I would probably stay away from bendamustine if I thought any patient were a potential candidate for CAR T-cell therapy.
CHAND: Have you used single-agent polatuzumab?
BUDDE: I have used polatuzumab plus rituximab and omitted the bendamustine.
With all these new agents—sometimes there are insurance coverage issues, but if the loncastuximab were covered by insurance—would you be willing to use loncastuximab in the second-line setting for patients who decline transplant?
CHAND: Isn’t that something [used in the third line or later]? For the previous patient, they had 2 prior lines of treatment, so I guess that would be an option. In the second line, if the patient is eligible for transplant, you give chemotherapy. Otherwise, you can use tafasitamab plus lenalidomide. But for polatuzumab or loncastuximab, the patient needs to have received at least 2 prior lines of treatment.
BUDDE: That’s correct. There are patients who have received prior loncastuximab treatment and [are then] moved on to subsequent CAR T-cell therapy, but the number [of such patients] is quite small. There is a concern about using CD19-targeting agents prior to CAR T-cell therapy. The [challenge] is deciding how to sequence these treatments, so [this decision is influenced by] whether you would consider CAR T-cell therapy in the future.
CHAND: What about sun exposure and loncastuximab? Are patients at high risk for a rash or [anything else]?
BUDDE: Yes. There is a high risk of skin toxicities because [loncastuximab] contains a payload, so it’s not a pure immunotherapy, it’s an antibody-drug conjugate.
YEH: For the trial of polatuzumab, bendamustine, and rituximab vs bendamustine and rituximab alone [NCT02257567], the median overall survival among patients who received the triplet was only 12.4 months [vs 4.7 months for patients who received the doublet] [HR, 0.42; 95% CI, 0.24-0.75; P = .002].1 That seems to be quite short compared [with] the [values reported in] the L-MIND trial [NCT02399085]2 of tafasitamab plus lenalidomide and the LOTIS-2 trial [NCT03589469] of loncastuximab tesirine.3
BUDDE: Yes, but it’s difficult to compare the absolute numbers between trials because the trial designs and patient populations are different.
YEH: The trial of polatuzumab, bendamustine, and rituximab1 is more in line with the L-MIND trial3,4 because [the patients had all received] 1 or more prior lines [of therapy]. [In contrast], in the LOTIS-2 trial, patients had received 2 or more prior lines of therapy.3 It seems that for a patient who has failed 1 line of therapy, the choice is the tafasitamab vs the combination of polatuzumab, bendamustine, and rituximab, but if a patient has had more than 2 lines of therapy, then loncastuximab can come into play. But I like the loncastuximab. It sounds like these are very heavily pretreated patients, CAR T-cell therapy recipients, and patients with double hits. These are the patients I have more trouble treating.
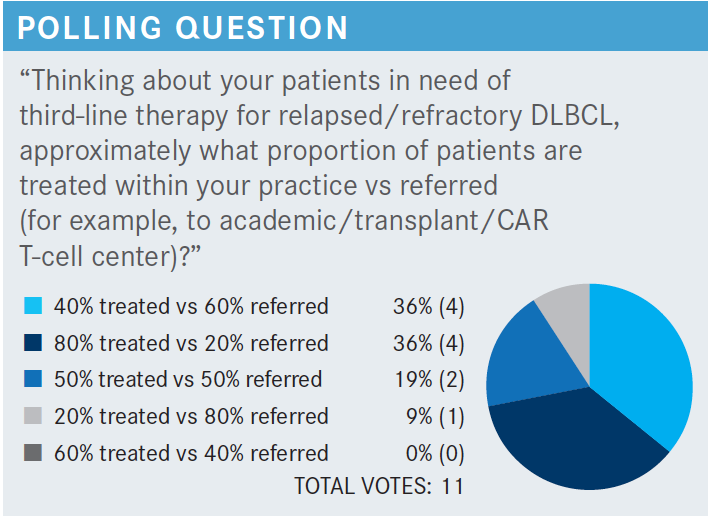
CHO: Would tafasitamab still work if the patient has lost CD19 [expression]?
BUDDE: As you know, most of our patients are allowed 1 prior line of therapy, and the current standard-of-care doesn’t involve any CD19-targeted treatment,5 so the [likelihood] of a patient having CD19-negative disease is very small.
CHO: What if you have to use it in a later line? Should you do a biopsy?
BUDDE: Yes, I think so. [We also need to consider], in the later-line therapies, will we see a similar response rate and duration of response in patients who are more heavily pretreated? I don’t think we know that yet.
CAO: For the polatuzumab trial, why was the polatuzumab stopped at cycle 6?1 Why was polatuzumab not continued until disease progression?
BUDDE: This is still a chemotherapy-based treatment, and it is difficult for patients to tolerate it beyond 6 months. One-third of the patients had to discontinue treatment because of adverse events.1
CAO: For the combination of polatuzumab, bendamustine, and rituximab, the main toxicity is from the bendamustine. I gave the doublet of polatuzumab plus rituximab to an 80-year-old patient for 6 cycles, and she tolerated it well. [If you were using the triplet regimen], you could drop the bendamustine after 6 cycles and continue with polatuzumab plus rituximab until disease progression.
BUDDE: Yes, you could try [that], but the peripheral neuropathy could also be a concern.1 Sometimes I will omit bendamustine if I think a patient cannot tolerate it well.
REFERENCES
1. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
2. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958
3. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:10.1016/ S1470-2045(21)00139-X
4. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
5. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 2.2022. Accessed April 18, 2022. https://bit.ly/3wge9yh
