Roundtable Discussion: Ademuyiwa Covers the Benefits and Challenges of Using Sacituzumab in Certain Populations of TNBC
During a case-based roundtable event, Foluso Olabisi Ademuyiwa, MD, MPH, MSCI and a group of peers discussed the benefits and challenges with using sacituzumab govitevan to treat a 48-year-old patients with triple-negative breast cancer.
Foluso Olabisi Ademuyiwa, MD, MPH, MSCI


RAZAQ: So sacituzumab govitecan [Trodelvy] has a very rapid response rate and a very high response rate. I know this drug is moving in the front line now, and there are some trials with new entrances in the adjuvant setting, too. Therefore, if somebody is progressing rapidly, I prefer to use sacituzumab in those patients. The adverse events profile is well known as we have all used it, so we cannot get it in the front line, but I’m hoping that we will be using it in the frontline setting soon.
ZOGHBI: I just want to say that I changed my second-line therapy once sacituzumab came in and became more available because I feel that this is a different mechanism of action to our typical chemotherapy.1
I usually use chemotherapy depending on the patient’s status [and any potential] comorbidities.
I do not have [a one-size-fits-all] plan, but I do agree that I like, after progression on those dose-dense adjuvant chemotherapies, to use carboplatin and either single-agent or one after the other depending on the disease burden. With sacituzumab being on the market now, I’m very interested in using it more and more up front, even for outpatient first-line therapy.
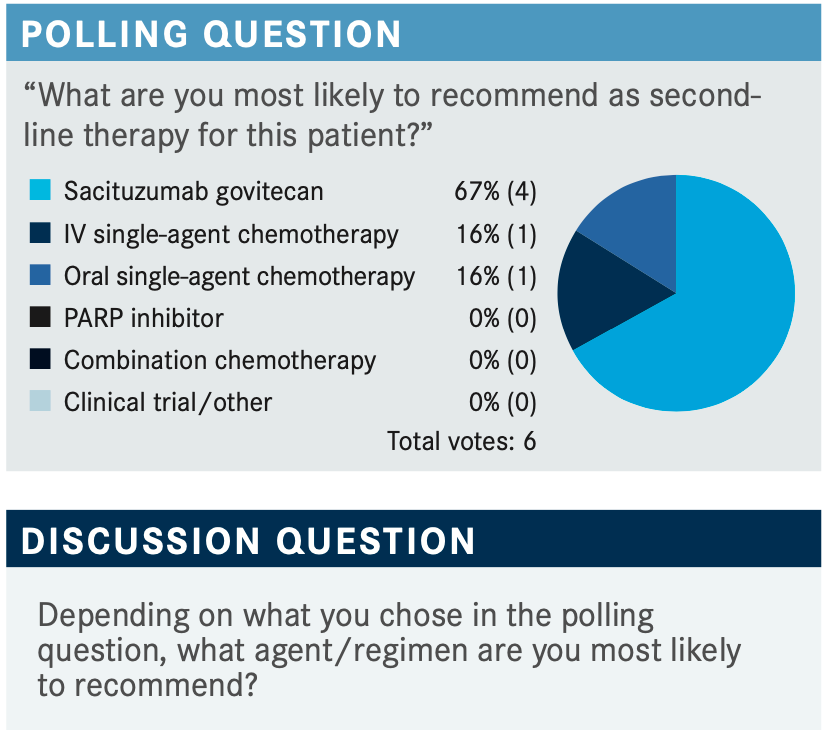
HADDADIN: With the prior exposure to treating with drugs and the rapid response, I think we can make a case of starting with it. However, it depends on the insurance. They might approve it or they might not, but whether you use combination or single therapy depends on the extent of the disease and how it affects the vital organs. Again, I do agree that we have to look at what are the best chances of response. If it gives you 30% response it might be better than the other available drugs, and if we can make an argument that they did receive treatment before then, sacituzumab might be the way to go. Also, if insurance approves it I think this is where we need to go.
ADEMUYIWA: I’m using sacituzumab more in the second-line setting, just based on the activity that was reported in the ASCENT [NCT02574455] trial.2 I’m reaching for this now in the second line and you are correct that there are studies looking at [sacituzumab] in the frontline setting, but right now, the approval is second-line and beyond.
GUPTA: So the drug has a unique mechanism of action and we like to use it even in earlier lines of setting. That’s [our motivation] given its excellent response rate. Now, I’m just curious, these patients in this trial they were heavily treated though, right? So the argument is that this drug was still active after 5 median lines of therapy and unless we have survival data, you cannot make an argument that we should use it earlier.
Although, I am in the camp that would like to use it earlier given a high response rate, but it cannot be simply stated. The drug costs a lot of money and we are not factoring that into the conversations here and, in my experience, there’s a high risk of neutropenia with these drugs. I think people like oral capecitabine so much better...this is the dissenting opinion, and while the drug is effective it is toxic. And maybe the toxicity that we saw in the trials is because the patients were heavily treated and were forced with so much chemotherapy, but I have used it in a couple of patients and the toxicity is high. [Right now it is exciting,] but in practice it’s not. Also, I do not know how to use a growth factor with this drug with the way it is scheduled. Are there any thoughts on that?
ADEMUYIWA: I have used it and I do agree with you that there is some notable toxicity with neutropenia and diarrhea. However, my personal practice is to give growth factors for all patients. What I do is I stick with the day 1 and 8 schedule, and patients come for day 1 then they come for day 8, and on their day 8 I give them pegylated filgrastim. So that by the time they’re coming back for the next cycle day 1, their counts would be optimal and I’ve been able to keep patients on a schedule by giving them growth factors. I use growth factors pretty much right off the bat with all these patients. Concerning the diarrhea, I’m very proactive about managing the diarrhea and I [tell my patients that] if they have more than 3 or 4 bowel movements they should call me, but you should be very proactive when using antidiarrhea agents. For certain patients, if I think the toxicities are more exaggerated, then I certainly check the UGT1A1; if it’s a different variant, those patients can have exaggerated toxicities. Managing the toxicities up front and being very proactive allows you to keep patients on schedule. I don’t know if anybody else has any comments about their practice.
HADDADIN: In my experience I do the same thing. I didn’t have any problems with diarrhea or neutropenia, but I use [granulocyte colony stimulating factor]. I think my patient’s main complaint was hair loss, and this is what they were not happy about, but other than that, the patient did well with the treatment.

HADDADIN: I use sacituzumab in the clinic in my patients who were heavily pretreated. The main 2 things we stressed about were diarrhea and neutropenia, but I didn’t have a problem with these. The response that [my patient] got was very brief but I think on the first count she progressed, but she was heavily pretreated, and the cancer did not respond to treatment.
ADEMUYIWA: What line was this that you used it in her? Can you remember?
HADDADIN: So this probably would have been about the fourth line because she went on a clinical trial at [The University of Texas] MD Anderson Cancer Center and this was [in the] fourth line.
ADEMUYIWA: Any other comments, particularly about efficacy, and how other people counsel patients regarding the mechanism of action specifically?
HAPANI: I have not used it but I was able to gather good information from people who have already used it. The focus [for patients] is going to be on diarrhea, neutropenic fever, and neutropenia. I am from India originally, and 2 to 3 months ago I started getting a lot of phone calls that people are wanting to get this drug but they could not get it. I have not even used it yet and they’re calling asking to get sacituzumab, but while patients need it there it’s not available. So the drug has picked up a lot of hype right now and once appropriate patients come through, I’ll be excited to use it and help them out.
ADEMUIYWA: With the phase 3 ASCENT trial in the context of other therapies that have been used in the setting of relapsed or refractory TNBC, I still have used sacituzumab routinely now. Moreover, if there’s no clinical trial for the patient, I’m reaching for this in second line as I’m compelled by the efficacy data. Just so you know, the options for patients include antimetabolites, vinorelbine, gemcitabine, and it sort of was much better than treatments of physician’s choice.
But I think certainly, there are toxicities; with proactive management of toxicities with growth factors, antidiarrheal agents, and sometimes patients have flushing so I use atropine for that, and I’ve been able to get patients through that.
I am using it now pretty much routinely in second line. In terms of my approach to molecular testing, at the time of relapse I am sending off molecular testing for genetic profiling to see if they would have the option of using immunotherapy in the first line, and I’m getting this routinely for all patients. I’m also getting germ-line testing for all patients if they haven’t had it in the earlier-stage setting.
Does anybody else have any comments about their approach to molecular testing or what line they see themselves using sacituzumab? Then for patients who got the therapies, the treatments of physician’s choice, how does that inform how you use sacituzumab vs reaching for a different chemotherapy?
HADDADIN: I think the standard right now for [a patient with] TNBC [is that] we’re going to be checking for their PD-L1 status and we’re going to be checking for both BRCA 1 and 2 aswell.
ADEMUYIWA: When are you checking for that?
HADDADIN: At the beginning. [For patients with TNBC] I figured it’s worth looking at the PD-L1, BRCA 1 and BRCA 2. Then you make your decision based on what they are scanning for [to decide on which] inhibitor to use. Are they scanning for it plus [giving chemotherapy at the same time]? Or are we going a different route, which is more chemotherapy?
ADEMUYIWA: So you do your genomic testing on all your patients?
HADDADIN: Yes.
ADEMUYIWA: The other topic to discuss is what we have learned [so far]. And if we have learned anything about other biomarkers to use as agents it’s the Trop-2 antibody-drug conjugate. Trop-2 is big with us in [treating patients with] breast cancers, particularly in TNBC.

ADEMUYIWA: Like I said for this in the third line, therapeutic options discussed were eribuilin, platinum and acitabine, and if they haven’t received that I’m not using a lot of those but there are a lot of toxicities with that agent. Clinical trials are certainly a factor at this point, but also at this point, of course, the goals of therapy are palliative. For most patients I think palliative is the most important to have control of symptoms, but obviously disease control and disease survival is also a goal of therapy. In most heavily pretreated patients, most of the time I advise them that palliative is what we’re looking for and then what influences decision-making. Survival of course is most meaningful but overall survival after having been pretreated, I think, is difficult to achieve. So PFS and clinical benefit rates are what I’m counseling patients on. Then, of course, safety and tolerability because these patients have been pretreated.
So I want to hear from others what other factors influence their decision-making and the goals of therapy for patients who have received 1 or 2 lines of therapy already. Anybody else, what they’re looking at when they’re recommending third-line options to patients with TNBC?
HOPKINS: It’s going to depend on the patient and what they want to do as far [as dealing with] toxicities, for me. I talk with the patients about options, and which ones have less toxicities and how often they have to come in, because sometimes that’s a factor as well.
ADEMUYIWA: What are you using in the third line for patients?
HOPKINS: Well, for most it [I would use] eribulin as it has less toxicity for them. In the second line I would mostly use carboplatin-gemcitabine especially if they’ve still got a good performance status.
ADEMUYIWA: Have you had a chance to use sacituzumab yet?
HOPKINS: No, I have not. That’s why I didn’t chime in because I haven’t had the chance to use it yet.
ZOGHBI: I just want to mention that I haven’t used the drug yet, but I feel that the toxicity of the drug [is a challenge], but of course there will be a learning curve like any drug that we’re going to use in the community. However, I feel the toxicities are something hopefully to a certain degree we will be able to manage. For example, one of the toxicities with other drugs that would concern me more is pneumonitis. That could be so bad with the patient even when they stop the drug. But hopefully, and again I haven’t had the experience with the diarrhea and the neutropenia, we have growth factor support, so we have more supportive measures now.
I still think the benefit will outweigh the risk with the survival of the drug and the good data still outweigh it, too, and there is no sequence of drug that fits all. We have just the individualized treatment for the patient [based on their characteristics and] what they want to do.
In doing clinical research it sometimes feels like in the community you have a different type of TNBC, such as patients that are PD-L1 negative, but for example, I have a patient who is almost blind that elected for no chemotherapy except the low dose. She stayed alive at 3 years on half the dose and she did well for 3 years presenting with stage IV TNBC. You have on the other hand patients who go through 1, 2, 3 [lines of therapy and] she didn’t even respond to the drug and had rapid disease progression. So I feel there is still an unmet need how to treat those triple-negative patients. And how even when we just say triple-negative they are BRCA negative, PD-L1 negative, and I still feel there is maybe more things we need to dig more to stratify patients and find the right treatment for them.
ADEMUYIWA: Yes, you are certainly correct; every biology in triple negative is so different. I think probably in the future, we’re going to be doing more molecular profiling and treating patients according to whether they’re the triple-negative subtypes that are coming out now of research—the basal subtype vs the immunomodulator tree vs the antigen receptor–positive subtype.3
References:
1. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. April 8, 2021. Accessed April 14, 2022. https://bit.ly/3M4ZcVZ
2. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021 Apr 22;384(16):1529-1541. doi:10.1056/NEJMoa2028485
3. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020 Jun 9;22(1):61. doi: 10.1186/s13058-020-01296-5
