Roundtable Discussion: Graff and Participants Debate Treatment Choices in TNBC
During a Targeted Oncology case-based roundtable event, Stephanie L. Graff, MD, discussed the case of a patient with triple-negative breast cancer who progressed following adjuvant chemotherapy and frontline carboplatin treatment.
Stephanie L. Graff, MD (Moderator)
Director of Breast Oncology
Lifespan Cancer Institute
Rhode Island Hospital
Providence, RI


GRAFF: My follow-up question to [this poll] is, if you chose chemotherapy: What are you most likely to recommend? What combination do you use? What single agent do you use? What’s your go-to?
ALI: Can we use sacituzumab govitecan [Trodelvy] in this situation after 1 [prior therapy]?
GRAFF: Not technically. I think you can probably appeal that with somebody’s insurance. But the label for sacituzumab govitecan is after 2 lines of therapy, at least 1 in the metastatic setting. Although she has had a very short recurrence, she hasn’t had any therapy in the metastatic phase.
EASAW: [Because] she is only 8 months out from adjuvant therapy, I would probably start her on eribulin [Halaven]. That would be one of my choices.
GRAFF: I like eribulin, too.
KAPPEL: She’s already had a couple of the good drugs, in terms of a taxane and dose-dense AC, and if she is clinically stable, maybe something like capecitabine.
VARADI: I would start, if it is monotherapy, probably gemcitabine. If she has significant visceral disease maybe carboplatin and gemcitabine.
GRAFF: I do like platinum therapies in this situation, too. I feel like in a post-KEYNOTE study world, more of our patients are going to be getting platinum in the neoadjuvant phase, [and concerning] this particular question the patient hasn’t had a platinum therapy yet.

GRAFF: In an alternate scenario, what frontline therapy [would we give or] what would we do differently if it had been a longer disease-free interval? The patient had a disease-free recurrence of 24 months before she had a recurrence. Would that alter your decision? Would you change your answer in that situation?
DAI: In this setting, I would use the paclitaxel. Previously I chose the capecitabine or oral chemotherapy. I thought it would give her a better quality of life and avoid hair loss. [In particular], she was just 8 months from previous adjuvant chemotherapy with the paclitaxel. Yet, in this scenario, 24 months after adjuvant chemotherapy I would have preferred to use paclitaxel again.
GRAFF: You can make the case that she doesn’t have paclitaxel resistance with a longer time in. Also, I like your comments about her alopecia. She probably has full hair regrowth in 2 years and giving her capecitabine or single-agent taxane if she could keep her hair would be patient-centered.

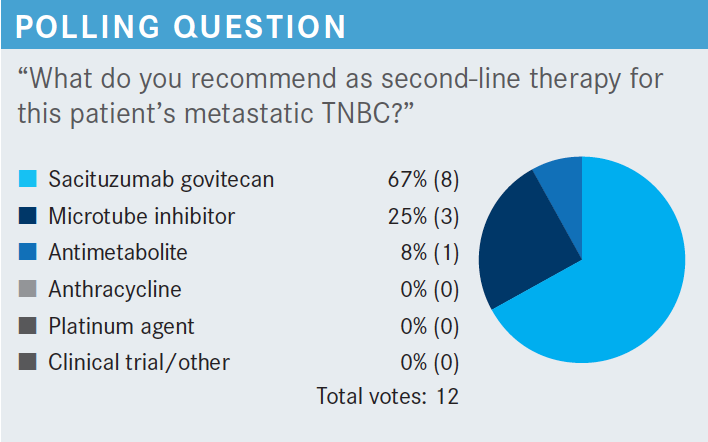

KAPPEL: [Will we] talk about the interval between 8 and 24 months? The other issue is how much disease they have, do they have a high tumor burden or a low tumor burden, and how symptomatic they are. We’ve gone way beyond the day of lung metastases or liver metastases and have to treat them aggressively, regardless of how symptomatic they are. I’m old enough to remember even when they were hormone receptor positive, if you had lung or liver metastases, you had to go to chemotherapy. Those days are clearly over. You focus on this 8 and 24 months and clearly that is important, but we also have these other variables to look at. How much disease, how symptomatic—things like that are helping us to choose between single agents or combination agents—and molecular profiling to see what else they’re eligible for.
DAI: The quality of life and treatment-related toxicities [are considerations].
GRAFF: I feel like peripheral neuropathy is definitely a factor for me because it tends to be the adverse event [AE] that I am bouncing back and forth between. If I just gave a drug that was causing a patient a lot of peripheral neuropathy, I would be very careful not to use the next line of therapy that is going to augment that.
ALI: I think platinum therapy is excellent and platinum should also be incorporated in the treatment in TNBC.
GRAFF: This case is BRCA negative. But in the BRCA-mutant population, or deleterious germline mutation population, it is less clear where the value of platinum lies in a patient who received a PARP inhibitor. So it gets complicated in the triple-negative space trying to sort out sequencing and order, but at some point in the mix it belongs in our list of medicines we turn to.
GUNDAVARAM: How about tumor mutation burden, have you used that for PD-L1–negative [patients]?
GRAFF: Definitely. We have the tissue-agnostic FDA approval for pembrolizumab [Keytruda] for patients that either are tumor mutation burden high or who are otherwise candidates for immunotherapy independent of PD-L1 expression.1 I definitely think that that is a consideration for our patients. I also do genomic sequencing on my patients with TNBC early on to look for options for clinical trials. We still don’t, as breast cancer oncologists, have the depth of options that our thoracic oncology colleagues have in terms of actionable mutations. I am still fantasizing about finding an NTRK mutation in a patient with breast cancer. I think we need to continue to explore those opportunities and look for trials for our patients. I will continue to scan what’s out there and certainly explore the next thing. Hopefully someday we will get to HER2-low disease.
KAPPEL: Unfortunately we are still behind our thoracic oncology friends in actionable markers. But you have X number of drugs for ALK-positive disease, and I probably had only 1 ALK-positive patient in the last [I don’t know] how many years. But the one ALK-positive patient that I finally had in the last few months had a tremendous response on his first PET scan.

GRAFF: For those of you who have used sacituzumab, tell me a little bit about your experience.
BRAUNSTEIN: In my experience, I have seen patients who have had metastases that have responded but occasionally needed to be admitted for neutropenia.
HUSSEIN: I have used it 3 times. One time there was evidence of very rapid progression after starting the drug, less than a month and a half. The other 2 times, the patients—even though they were relatively young and in good shape overall—had severe fatigue, so we had to switch to something else. It was tough to tolerate.
GRAFF: Did you dose reduce for fatigue?
HUSSEIN: I did. The other thing also is that neutropenia was very strong, and I was surprised that there is no recommendation for having something like pegfilgrastim [Neulasta] built into the treatment protocol. So I typically add that now after day 8. It depends. I tend to give them filgrastim [Neupogen] after the first day.
GRAFF: I give patients that I have prescribed sacituzumab [to a dose of] pegfilgrastim on day 9 or so now, as a part of my standard template. Luckily, in the United States, I do not have a problem getting coverage for it. I have experience speaking to international oncologists about sacituzumab and it is a much bigger deal internationally to get growth factors sensitive to the bigger barrier to the international use of sacituzumab govitecan, with the diarrhea. For patients that have more immediate-onset diarrhea, the recommendation is to use atropine very quickly, even in the infusion area if they have rapid-onset diarrhea. For patients who have delayed-onset diarrhea, it is loperamide [Imodium] and then escalation to whatever your next favorite antidiarrheal is, and then adding on as you need to with dose reduction.
The dose-reduction label for sacituzumab is a pretty brisk dose reduction. It is a 25% dose reduction followed by 50% dose reduction.2 I think a lot of us are conditioned to do the 10% to 15% dose reduction, but sacituzumab isn’t a drug you mess around with, so do a pretty aggressive 25% to 50% dose reduction for patients that are having severe AEs, as per the label, and per the original ASCENT trial [NCT02574455].2,3 For patients that are having a lot of diarrhea, sometimes a longer break to allow for recovery, correction of electrolytes, rehydration, even a course of physical therapy has helped my patients recover from fatigue. I found that sometimes the fatigue is just, for lack of a better word, the emptiness of having had diarrhea that was more pronounced.
Counseling my patients about sacituzumab govitecan is probably similar to my experience of counseling patients about abemaciclib [Verzenio] or capecitabine, which is that diarrhea is something that I want you to tell me about. In my experience, there is always that 1 patient out of 5 patients that feels like diarrhea is this necessary evil to cleanse their body of the evil toxins, and they do not call you. I don’t know [why] they will sit at home and have diarrhea or 5 or 6 watery stools for days on end, and then end up hospitalized before they call and tell you that they are having diarrhea. So I have a pretty brisk, big talk with my patients when I prescribe these more heavy-diarrhea chemotherapeutics about how important it is that I hear about it with their first loose stool because it is not healthy, and we need to be in front of any symptoms that they develop. I think it helps them to understand that I am worried, because it helps promote the fact that it is OK for them to call me and call my nurses. What other thoughts do you have?
KAPPEL: Diarrhea is clearly a significant toxicity that people end up in the hospital from. So you have toxicities like neuropathy that inhibit patients’ quality of life. You have diarrhea that can put these people in the hospital. I’m old enough to remember when irinotecan came out and it had the initial presentations at the Mount Sinai Conference every year, and everybody was talking about diarrhea and irinotecan and the initial fear factor of diarrhea. It’s a big toxicity that people don’t stress enough. It’s significant for a lot of drugs.

ALI: I use it in second-line treatment. [Sometimes] you are doing [first-line] treatment and then treatment in the metastatic setting, so then sacituzumab will be third line.
EASAW: Having never used it and listening to the AEs, I am a little hesitant about bringing it more upfront because of all the treatment options we have. I mean this is an incurable disease, and the other options we have, like eribulin or anti-BRCA [therapy,]…seem to have a better toxicity profile. Because this is somebody who is not going to be cured, quality of life is probably most important at this point, unless we come up with a strategy to minimize the AEs. I was very excited about it when it came out, but then when I listen more about the AEs, it is a little scary to try on somebody who [has only been treated with chemotherapy].
GRAFF: Yes, but I think talking about survival is important. Most of the drugs used in metastatic TNBC don’t [result in long] survival. We have a survival end point for eribulin and we have a survival end point for sacituzumab govitecan, and nothing else that we use.
Everything we use has toxicity, so when you balance toxicity vs outcome, I think most patient advocates that I have worked with would chose diarrhea and 6 more months than no diarrhea and 1.7 months.
It’s a complicated decision, and I think that’s what happens in the exam room, right? That’s what shared decision-making looks like. I don’t think any of you should sell yourselves short on your ability to manage chemotherapy AEs. You are all experienced oncologists and I know you can all do that. Keep in mind that in the ASCENT trial, only about 20% to 30% of the patients received growth factors.3
Again, in my practice, I’d say 90% of my patients received growth factors out of the gate with sacituzumab so that I can keep them away from those statistics that we saw in the ASCENT trial about the rate of grade 3 neutropenia. And with growth factors, I am not anywhere near that statistic for grade 3 neutropenia. And you do aggressive dose reductions.
KAPPEL: Breast cancer is a disease in which these patients, even with TNBC, may live longer than patients with lung cancer, and maybe some patients with colon cancer. Now, OS [overall survival] in these clinical trials is a very hard marker to get to.
I think we’ve all accepted PFS [progression-free survival] as a surrogate marker to OS in breast cancer. Otherwise these studies would never get done, and you could never get the math to work given the numbers of patients that you’d have to recruit to get the studies done. I do feel that PFS is an adequate surrogate marker here.
My follow-up question would be in what line of therapy do you order biomarker testing? Do you order it right away in the metastatic setting? Do you wait until after the first line or second line? The first thing I ask drug representatives when these new drugs come out is do the mutations change? Do they evolve? Are these mutations always there or do they evolve over time? And what is the appropriate time to get these biomarker testings?
GRAFF: In TNBC, I tend to order genomic profiling, whether that’s Caris [testing], FoundationOne [CDx], or Guardant[360 CDx], in patients on their biopsy to confirm metastatic disease, assuming I have enough tissue after confirming that it is still triple negative.
A large part of that, for me in practice, is because I am already [checking] PD-L1 expression so I know what category I am in, and because I have a huge clinical trial portfolio, so I am trying to match them to the right trial. In the real world, we still don’t have any great targeted therapies for TNBC. We can arguably call sacituzumab govitecan a targeted therapy, but we don’t measure the target.
We give it to all comers of TNBC. And we have PD-L1 but we don’t need genomic sequencing for that. We can do it a lot of times at immunohistochemistry in our local labs, or whatever your pathologists are using. For BRCA1 and BRCA2, we are doing germline testing, not genomics. So in the triple-negative space I am doing it more as a clinical trial list than as what’s commercially available. For estrogen receptor–positive breast cancer, I have been in the habit of profiling patients after progression on CDK4/6 inhibitors because I have had a large number of [patients in] the ESR1 mutation trials and you are more likely to develop an ESR1 mutation after exposure to endocrine therapy, so I want to catch it at the end of that CDK4/6 exposure.
But in truth, some patients likely have that after even just their adjuvant exposure to aromatase inhibitors or tamoxifen [Soltamox]. So especially as you near the typical shelf-life of your CDK4/6 inhibitor, it may be reasonable to do a genomic profile. In HER2-positive breast cancer, I still don’t know the answer to that. There are very limited data because there are so many drugs right now in the HER2-positive space, and what prompts us to treat HER2- positive breast cancer is HER2, not PIK3CA or anything else that we know about. So we are not doing it.
CHEUNG: Could you break down the response between rapid positive vs rapid negative patients?
GRAFF: They did not break down the results by BRCA negative or BRCA positive, as far as I remember. So no, I don’t think so. They broke it down by Trop-2 expression. There’s no clear evidence.
EASAW: [Regarding] your previous statement when somebody relapses or progresses on a CDK4/6 inhibitor, would you rebiopsy that or just get a mutation panel? Or how about looking at biopsies?
GRAFF: I will often rebiopsy them then. And if they have bone-only disease, sometimes I will send a blood-based assay in that situation. Because with a bone-based disease, by the time pathology is done decalcifying it, I can’t get what I want.
EASAW: So going back to the bone-only disease…[if it was positive, and] we do a biopsy, you can get a negative hormone receptor status. So would you treat that as triple negative at that point or are you recommending giving hormone therapy at that point?
GRAFF: I’d have a conversation with my pathologist about whether or not they believe that it’s triple negative. Ninety-nine percent of the time I do not treat known hormone receptor– positive breast cancer with bone-only metastasis that shows loss of expression on a bone biopsy as a change in marker. I consider it a fault in the technique. I need more compelling evidence, because that’s not the typical story of a TNBC, that it’s bone only and it progresses and it stays bone only.
REFERENCES
1. Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2022. Accessed April 1, 2022. https://bit.ly/3knVCur
2. Troldevy. Prescribing information. Immunomedics, Inc; 2020. Accessed April 1, 2022. https://bit.ly/37UpR9F
3. Bardia A, Hurvitz SA, Tolaney SM, et al; ASCENT Clinical Trial Investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541. doi:10.1056/NEJMoa2028485