Lee Discusses Current Landscape in Management of Clear Cell RCC
During a Targeted Oncology case-based roundtable event, Chung-Han Lee, MD, PhD, discussed the use of approved combination regimens including tyrosine kinase inhibitors and immune checkpoint inhibitors for a patient with clear cell renal cell carcinoma.
Chung-Han Lee, MD, PhD
Medical Oncologist
Memorial Sloan Kettering Cancer Center
New York, NY

Targeted OncologyTM: What are the current recommendations for intermediate- or poor-risk patients with relapsed or stage IV clear cell RCC?
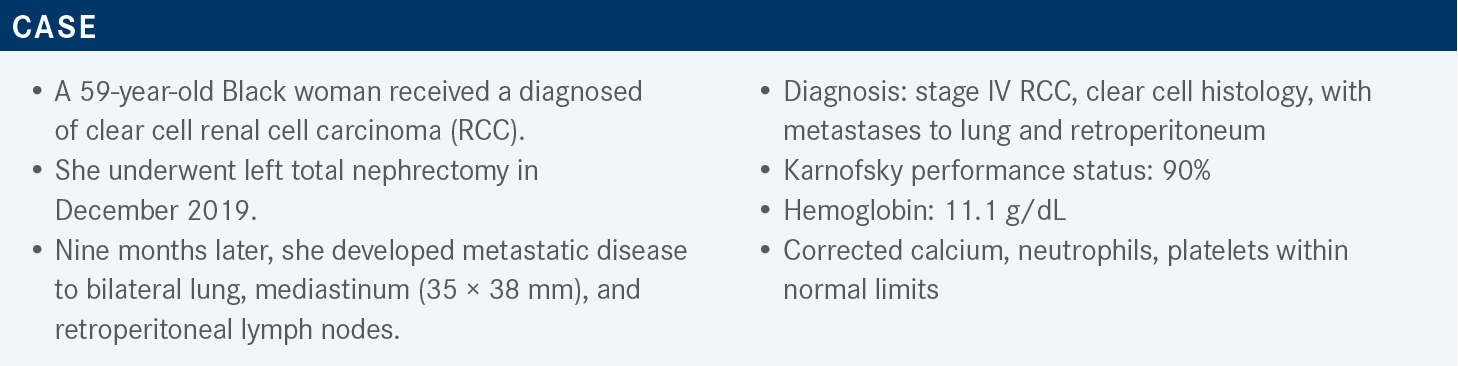
LEE: Based on the most current version of the National Comprehensive Cancer Network guidelines for a patient who has intermediate- or poor-risk disease, there are still multiple regimens that are approved and had category 1 evidence.1 Three of which are TKI-IO [tyrosine kinase inhibitor and immunotherapy] combinations, including axitinib [Inlyta]-pembrolizumab [Keytruda], cabozantinib [Cabometyx]-nivolumab [Opdivo], and lenvatinib [Lenvima]- pembrolizumab. Ipilimumab [Yervoy]-nivolumab also has category 1 data in this setting, and cabozantinib monotherapy is a preferred regimen without category 1 data, based on the CABOSUN trial [NCT01835158].
How do the data compare across all these treatment regimens?
If we line up most of the TKI-IO combinations or the IO-based combinations right now, there are certainly multiple regimens that can be compared. Of course, we have the caveat [that] we can’t do a cross-trial comparison between them. Ipilimumab-nivolumab has had the longest follow-up and demonstrated an improvement in overall survival [OS] and objective response rate [ORR] compared with sunitinib [Sutent].2,3 In the intermediate- and poor-risk populations, if we look at the intent-to-treat population, the results certainly were outpowered. Primary progression of disease [PD] rate was seen at approximately 18% for [individuals] on ipilimumab-nivolumab.
When we talk about TKI-IO combinations, we see fewer [individuals] having primary PD, with axitinib-pembrolizumab at 11%, cabozantinib-nivolumab at 6%, and lenvatinib-pembrolizumab at 5%.4-6 All these regimens have shown an improvement in progression-free survival [PFS] with significant P values2-6 and improvements in the ORR, ranging from 55% to 71%. [In some of these regimens], a median OS has not been reached based on the duration of follow-up, but the hazard ratios for OS benefit was seen with all these treatment regimens.
What is important to know about recently approved combinations?
Beyond axitinib-pembrolizumab, the 2 regimens that [received] regulatory approval in 2021 were cabozantinib-nivolumab and lenvatinib-pembrolizumab. Cabozantinib-nivolumab was FDA approved on January 22, 2021,7 and lenvatinib-pembrolizumab [received approval] on August 10, 2021.8
The lenvatinib-pembrolizumab was originally studied in the phase 3 CLEAR study [NCT02811861], which explored lenvatinib-pembrolizumab or lenvatinib-everolimus [Zortress] vs sunitinib.6 On this protocol, the key eligibility criteria included [individuals] with clear cell RCC, [and patients] had to be treatment naive with a KPS [Karnofsky performance status] greater than 70%. All of [whom] had to have measurable disease and adequate organ function. Patients were stratified based on geographic location and Memorial Sloan Kettering Cancer Center [MSKCC] risk criteria.
What was the design and results of the CLEAR study?
Patients were randomized 1:1:1 [to receive] lenvatinib plus pembrolizumab, based on the lenvatinib 20-mg dosing plus pembrolizumab 200 mg every 3 weeks for up to 2 years6; lenvatinib 18 mg and everolimus 5 mg, as per the FDA-approved dose based on the HOPE-205 trial [NCT03173560]6,8; or sunitinib at the standard 50 mg for 4 weeks on and 2 weeks off.6 The primary end point was PFS by independent radiographic review with RECIST 1.1, with key secondary end points of OS, ORR, safety, and quality of life.
This was a positive study [that] met its primary end point with a median follow-up of 26.6 months,6 where they demonstrated an improvement in PFS of lenvatinib-pembrolizumab vs sunitinib, with a hazard ratio of 0.39 and a P value of less than .001 [95% CI, 0.32-0.49]. Lenvatinib-everolimus also showed an improvement in PFS, with a hazard ratio of 0.65 and a significant P value [95% CI, 0.53-0.80; P < .001]. In comparison [with] sunitinib, there was an improvement in the median PFS from 9.2 months for sunitinib [95% CI, 6-11] to 23.9 months for lenvatinib-pembrolizumab [95% CI, 20.8-27.7].
When we look at some of the subgroup analyses, we can see that most of the subgroups that were looked at favored lenvatinib plus pembrolizumab, including looking at age, sex, geographic location—all the risk categories—both by IMDC [International Metastatic RCC Database Consortium] and MSKCC risk.6 Also, at performance status, the number of sites of disease, and regardless of whether [individuals] were PD-1 positive vs PD-1 negative [were also observed].
If we further look at this based on some prespecified analyses of adverse prognostic factors, they also looked at whether there was a benefit for lenvatinib-pembrolizumab vs sunitinib.6 And again, they ended up seeing benefits, including whether the patients had baseline bone metastases or liver metastases, stratification of PD-L1 status, and whether patients had sarcomatoid histology.
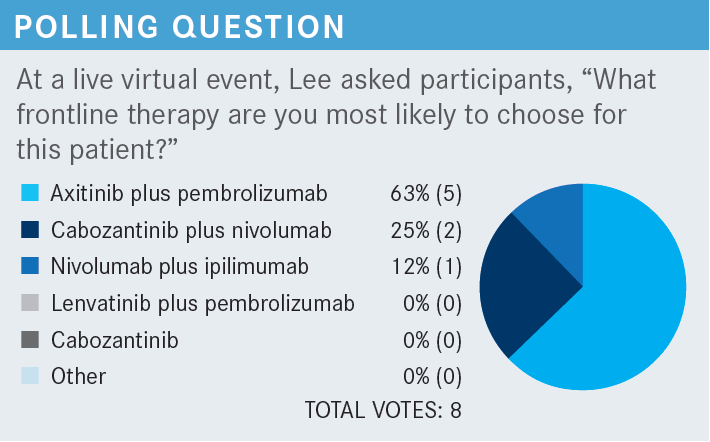
How do the survival data compare for the combination of lenvatinib and pembrolizumab?
When we look at the OS data, we see an early separation of the curves, where the lenvatinib-pembrolizumab survival curves separate quite early.6 We see a crossing of the curves later, beyond the 30-month mark, but based on the follow-up, that part of the survival curve certainly remains unstable. It’s unclear whether that will end up changing later. In terms of lenvatinib-everolimus, we see a quite similar OS, whether they were treated with sunitinib vs lenvatinib-everolimus up until about the 15-month mark, in which the sunitinib curve seems to be starting to separate from the lenvatinib-everolimus first-line data curve. In the 33-month follow-up, the median OS has not been reached and we continue to have a hazard ratio of 0.72 [95% CI, 0.55-0.93]. It’s still in the positive territory, moving up slightly, as we’ve seen with some of the other TKI-IO combinations with more standard follow-up.
If we compare ORRs, lenvatinib-pembrolizumab ended up showing a 71% ORR [95% CI, 66.3%-75.7%].6 Lenvatinib-everolimus was 54% [95% CI, 48.3%-58.7%] compared [with] sunitinib at 36% [95% CI, 31.2%-41.1%]. Relatively small numbers of [individuals] only had stable disease at 19.2%, whereas [stable disease for] the TKI without IO combinations or sunitinib by itself was well within the 30% range. With [individuals] with a primary progression of disease, there was a 5.4% primary PD rate for lenvatinib-pembrolizumab, 7.3% for lenvatinib-everolimus, and 14% for sunitinib.
One thing people often do talk about is the complete response [CR] rate. They were able to show a 16% CR rate with lenvatinib-pembrolizumab, 9.8% with lenvatinib-everolimus, and 4.2% with sunitinib—all of which were statistically significant. The median duration of response for lenvatinib-pembrolizumab was 26 months, lenvatinib-everolimus was 17 months, and sunitinib was 15 months. At the 24-month mark, 80% of [patients] who had a CR remained in CR in the intent-to-treat population, and [approximately] 74% who initially had a CR remained in CR at the 3-year mark at 36 months.
Are there notable toxicities for clinicians to be aware of?
In terms of thinking about the drug exposure for the patients, the median duration of drug treatment response was 17 months for lenvatinib-pembrolizumab,6 [11 months for] lenvatinib-everolimus, and sunitinib was 7.8 months. For treatment-emergent adverse events, as we have seen with most TKI combinations, most [individuals did] experience some [grade 3] treatment-emergent adverse events. Lenvatinib-pembrolizumab was 82%, lenvatinib-everolimus was 83%, and sunitinib was 71%. Many people also required some dose reduction within the TKI with close to 70% for [patients] treated with lenvatinib-pembrolizumab, 73.2% for lenvatinib-everolimus, and about half the patients for sunitinib.
Toxicities requiring the actual discontinuation of the TKI were [approximately] 26% for lenvatinib-pembrolizumab, 22% for lenvatinib-everolimus, and 14% for sunitinib.6 For pembrolizumab, [approximately] 29% of [patients] had to discontinue and 25% had to discontinue the everolimus. In terms of discontinuing both medications, [approximately 13%] had to discontinue lenvatinib-pembrolizumab and 19% had to discontinue lenvatinib-everolimus.
Lenvatinib-pembrolizumab was associated with slightly higher levels of grade 3 diarrhea and hypertension.6 Stomatitis was about the same, [whereas] hypothyroidism was slightly higher and was also higher compared [with] sunitinib. The amount of fatigue was about the same, hand and foot syndrome was about the same, and proteinuria was certainly higher with lenvatinib-pembrolizumab, along with rash.

What are your dosing recommendations for this regimen?
[There are also] more practical considerations for lenvatinib-pembrolizumab. The recommended FDA starting dose is 20 mg daily, [whereas] pembrolizumab is 200 mg every 3 weeks or up to disease progression for up to 2 years. [Individuals] will continue the lenvatinib beyond the 2 years as a single agent either for PD or toxicity. Lenvatinib is given in 4-mg and 10-mg tablets. The recommended dose reduction scheme is to go from 20 mg to 14 mg to 10 mg.
There is complexity with this regimen with regards to having 2 different pills that are available. Certainly, the process for getting dose exchange may not be as smooth as we would hope for. That’s something worth mentioning to the companies when you talk about this, because that’s a real-world regimen in which they have some degree of dose modification. It certainly should be up to the companies to try to provide this in a way that we can effectively follow their dose reduction scheme.
What other studies clinicians should be aware of?
The other study we have for a TKI-IO in this setting is CheckMate 9ER [NCT03141177], [which was a large, randomized phase 3 study that looked] at cabozantinib-nivolumab vs sunitinib. The key inclusion criteria are advanced or metastatic RCC with clear cell components, any IMDC group, [and patients] were randomized 1:1 [to receive either] cabozantinib-nivolumab or sunitinib. The primary end point was PFS and key secondary end points were OS, ORR, and safety.
From a PFS standpoint, the CheckMate 9ER study demonstrated an early separation of the curves, a hazard ratio of 0.51, and a P value of .001 [95% CI, 0.41-0.64].5 A difference in median PFS, with cabozantinib-nivolumab showing a median PFS of 16.6 months [95% CI, 12.5-24.9] and sunitinib at 8.3 months [95% CI, 7.0-9.7]. The updated 2-year follow-up showed the median PFS at 17 months vs 8.3 months and a hazard ratio of 0.52 [95% CI, 0.43-0.64]. From the subgroup analyses, everything tended to favor cabozantinib-nivolumab regardless of region, prognostic risk groups, PD-L1 status, age, gender, KPS, whether they had bone metastases, and whether they had prior nephrectomy.
For the secondary end point of OS, there was an early separation of the OS curves.5 At the initial presentation, they demonstrated a hazard ratio of 0.6 with a significant P value of .001 [98.9% CI, 0.40-0.89]. At the 2-year follow-up, the median OS was not yet reached by 29.5 months for the sunitinib arm, with a hazard ratio of 0.66 and a P value of .0034 [95% CI, 0.50-0.97].
They [also] showed an improvement in the ORR.5 Cabozantinib-nivolumab had an ORR of 55.7% vs 27% for sunitinib, [as well as] an 8% CR rate vs 4.6% for sunitinib. [A total of] 5.6% of patients receiving cabozantinib-nivolumab had primary progression of disease vs 13.7% of [patients] with sunitinib. The median time to response was relatively quick, essentially, at 2.8 months at the first cycle, with a median duration of response of 20 months for cabozantinib-nivolumab vs 11 months for sunitinib. At the 24-month follow-up time point, the ORR were fairly similar between the 2 arms, with a CR rate that did go up slightly to 9.3% vs 4.3% and a disease control rate of 88.2% vs 70%.
In thinking about some of the safety data they had before, more than half of [patients] required dose reductions on cabozantinib or sunitinib at 56% vs 51%; 44% required discontinuation of the drug on cabozantinib-nivolumab, with 27% of them being for disease progression.5 For toxicities leading to discontinuation or disease, it was 15% for either drug, 5.6% for nivolumab alone, 6.6% for cabozantinib alone, and 3.1% for cabozantinib plus nivolumab. At the updated follow-up at 24 months, the discontinuation rate was close to 10% for nivolumab, 7.2% for cabozantinib, and the combination at 6.6%.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 4.2022. Accessed April 21, 2022. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
2. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370-1385. doi:10.1016/S1470-2045(19)30413-9
3. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi:10.1136/esmoopen-2020-001079
4. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
5. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
6. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
7. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. News release. FDA; January 22, 2021. Accessed April 21, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-cabozantinib-advanced-renal-cell-carcinoma
8. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. News release. FDA; Updated August 11, 2021. Accessed April 21, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen