Tafasitamab/Lenalidomide Combo Demonstrates Favorable Efficacy in DLBCL
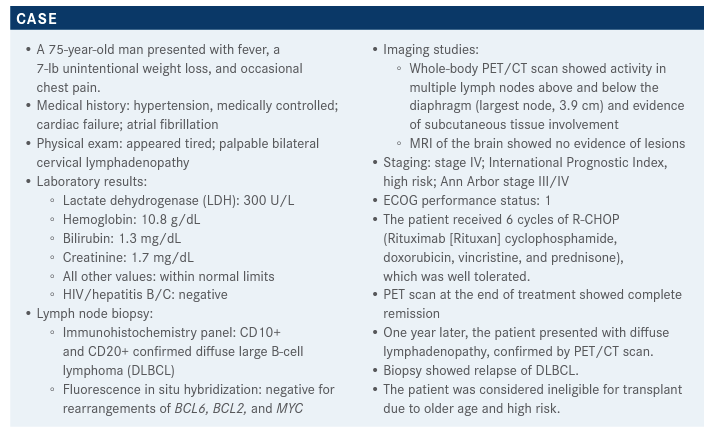
In the case that a clinical trial is not an option for a 75-year-old male patient with diffuse large B-cell lymphoma who is ineligible for transplant due to older age and high risk. Herbert A. Eradat, MD, explains his treatment plan.
Herbert A. Eradat, MD

In the case that a clinical trial is not an option for a 75-year-old male patient with diffuse large B-cell lymphoma (DLBCL) who is ineligible for transplant due to older age and high risk. Herbert A. Eradat, MD, an assistant professor of Medicine UCLA Health in Santa Monica, CA, explains his treatment plan.
Targeted Oncology™: What would you most likely recommend for this patient if a clinical trial is not an option?
ERADAT: I’ll review some of the data and put in context what the available options are for the relapsed or refractory patient with DLBCL and the recommendations from the various cooperative groups. In the NCCN [National Comprehensive Cancer Network] guidelines, the regimens are listed in alphabet-ical order, so there’s no preference recommended in the guidelines. GEMOX [gemcitabine (Gemzar) plus oxaliplatin (Eloxatin)] and rituximab [Rituxan] is certainly a reasonable strategy in the relapse setting as a palliative approach. The newer strategy of polatuzumab vedotin [Polivy], bendamustine [Bendeka], and rituximab, [or pola-BR], is also a good one.1 These are approved for DLBCL in adult patients who have progressed after 2 or more prior lines of therapy.
There are other chemotherapy strategies includ-ing tafasitamab [Monjuvi] plus lenalidomide [Revlimid]. Tafasitamab is a CD19-directed immunotherapy strategy that was approved in 2020 for relapsed or refractory DLBCL in patients who are not eligible for autologous stem cell transplant [ASCT].2 In certain circumstances, lenalidomide plus rituximab or ibrutinib [Imbruvica] are available for non–germinal center B-cell [non-GCB] DLBCL.1
What is the role of tafasitamab plus lenalidomide in DLBCL?
Tafasitamab was approved in combination with lenalidomide in July 2020 for patients with relapsed or refractory DLBCL and also patients who had low-grade lymphoma that transformed to DLBCL.2,3
Lenalidomide leads to activation of the natural killer cells. It also has some direct antiproliferative properties on the B-cell clone, but it activates T cells in a cerebral-mediated mechanism. It’s a form of immunotherapy and it was already approved for mantle cell lymphoma in the follicular and marginal zone. Many clinicians used it in combination with rituximab for DLBCL because it is certainly active in that context. In the L-MIND study [NCT02399085], lenalidomide was combined with MOR208, which is the original compound of tafasitamab, an anti-CD19 monoclonal antibody. So just as in CD19-directed CAR [chimeric antigen receptor] T-cell therapy, this is an antibody strategy.3
[Lenalidomide is] a model, modified antibody that increases antibody-dependent cellular toxicity and direct cell death. The single-agent activity was encouraging in patients with non-Hodgkin lymphoma with low-grade lymphoma, and long durable responses were observed in DLBCL, so this study was conducted. [L-MIND was a] phase 2, single-arm, open-label, multicenter study. [Investigators enrolled] patients who had relapsed on 1 to 3 lines of therapy or who were ineligible for [ASCT or high-dose chemotherapy]. They excluded primary refractory patients from enrollment.4,5
How was the L-MIND trial designed? What was the efficacy?
Patients received lenalidomide at a starting dose of 25 mg daily on a 21-day cycle followed by 7 days off. This went on for about 12 cycles, so 12 months. Tafasitamab is an infusion and there is a loading dose on days 1, 8, and 15 at 12 mg/kg for the first 3 cycles and then patients would go to day 1 and day 15 at the same dose [for the next cycles].3 Patients who had a response plus stable disease could continue tafasitamab as a single agent and stop lenalidomide after a year of therapy. Tafasitamab was continued until progression. The primary end point in this study was overall response rate [ORR]. The secondary end points were progression-free survival [PFS], durability of responses [DORs], safety, and overall survival [OS].4,6
The median age [of enrolled patients was] 72 years, with a range of 62 to 76 years. These were patients who were transplant ineligible typically because of age or comorbidities. Around 50% of patients had a high International Prognostic Index score of 3 to 5. Around 75% of patients had stage III or IV disease, so a heavy burden of disease. Over half the patients had elevated LDH. The median prior lines of therapy were 2 but ranged from 1 to 4. The eligibility for the study was 1 to 3 prior lines of therapy and there was a patient who had 4 prior lines of therapy who was excluded. About 50% of patients had received 1 prior line of therapy and around 50% had received 2 or 3 prior lines of therapy. During analysis, about 20% of enrolled patients were found to have refractory disease. The definition of [primary] refractory [DLBCL] is patients who did not respond to anthracycline, [which is frontline therapy,] or who had progressed within 6 months of anthracyclinebased therapy.6
The majority of patients, however, were refractory to the last line of therapy. So if they had received therapy beyond anthracycline, they were refractory to it. About 11% of patients had prior ASCT. For most patients, the cell of origin was not clarified but it included a mix of GCB, non-GCB, and unclassified or unknown.6
Response rates of the combination were assessed at 17 months and for patients who had durable responses [after 24 or more months of follow-up]. The ORR was around 60% whether you measured during therapy or long-term because patients maintained a response for more than 2 years. Complete response [rates] were around 40%, which is impressive. Around 20% of the patients had a partial response and another 14% had stable disease. So cumulatively, the clinical benefit or disease control [rate] is around 78% or 74%. Most impressive is the median DOR, which is around 21 months for those patients who were assessed at 17 months. With longer follow-up, the median DOR goes up to 34 months.6,7
The median PFS was 12 months, and the OS was not reported. At 18 months, the OS [rate] is around 64%, so most patients are still alive. At more than 2 years of followup, the PFS starts to go down. Remember, this is a patient population that has relapsed DLBCL, so eventually they will relapse. But at least in the 24-month follow-up period, a reasonable number of patients had durable responses.6,7
Do you still see similar results when you use tafasitamab after polatuzumab? What were the toxicities in the L-MIND trial?
I have not personally used it. This is an area that requires addressing because this is a CD19-directed strategy and we have a number of them available, such as tafasitamab, polatuzumab, and others. It’s unclear whether, for example, you can get a response with an alternative CD19-directed therapy when 1 has failed. Certainly, if the tumor loses CD19 expression then that’s understandable. But it’s unclear because this is also an issue of an immunomodulatory strategy. So if, for example, the lenalidomide/tafasitamab strategy stops working, an antibody-drug conjugate may be a different approach.
More than 20% of the toxicities were hematologic, including about 48% of those being grade 3 or grade 4 neutropenia. Most of the anemia is grade 1 to 2, and the same for thrombocytopenia. About 12% of the patients had grade 3 thrombocytopenia. About 10% of patients had febrile neutropenia, hence 90% of patients tolerated [treatment] without febrile complications. This therapy can cause some lymphopenia and 1% of patients had grade 4 agranulocytosis.3,6
Most common nonhematologic adverse events [AEs] tended to be fatigue, which is typical of lenalidomide-based strategies, followed by diarrhea, cough, and fever. There is a minor population of patients who developed peripheral edema. Infections, including respiratory tract infections, and anorexia are not unusual in this patient population.
Serious AEs [are those] that required hospitalization, treatment discontinuation, or led to death. About 13% of treatment-related AEs led to death, and AEs of special interest such as cytokine release occurred in 9% of patients.3,6
With lenalidomide, there were tumor flares and that’s something to be aware of, especially in the initial phase of therapy. The majority of AEs of special interest are tumor flares and rashes. About 12% of patients had to discontinue therapy because of AEs. Almost 45% of patients had to have 1 or more reductions in the lenalidomide dosing. It’s important to recognize that perhaps this is a high dose of lenalidomide, especially in elderly patients who have limited kidney function.3,6
The treatment protocol [for L-MIND was] the combination [with tafasitamab/lenalidomide for the first 12 months] and then the patients moved to tafasitamab monotherapy. A lot of the AEs were in the initial 12 months of therapy. Once the patients move to the tafasitamab monotherapy, which is after cycle 13, there were far fewer AEs. The majority of these AEs tend to be grade 1 and a few grade 2 events, but nothing significant. There were 5 patients that had grade 4 neutropenia [in the tafasitamab monotherapy phase]. Most of the patients tolerated the regimen well, especially the monotherapy phase.4
References:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 4.2021. Accessed June 6, 2021. https://bit.ly/3geoS5N
2. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed June 6, 2021. https://bit.ly/3bodb9v
3. Monjuvi (tafasitamab). Prescribing information. MorphoSys US Inc; 2020. https://bit.ly/2S38ZW7
4. Salles G, Duell J, González Barca E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Hematol Oncol. 2019;37(suppl 2):173-174. doi:10.1002/hon.130_2629
5. Jurczak W, Zinzani PL, Hess G, et al. A phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood. 2019;134(suppl 1):4078. doi:10.1182/blood-2019-124297
6. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
7. Salles G, Duell J, González Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020; Virtual. Abstract EP1201.