Roundtable Discussion: Gadgeel Leads a Discussion on the Timing for RET-Targeted Therapy in NSCLC
According to a poll, the majority of physicians would wait for molecular testing on a patient metastatic lung adenocarcinoma. Then, upon diagnosis of RET-mutant non–small cell lung cancer, a group of oncologists explained how they would go about treatment for the patient.
Shirish M. Gadgeel, MD

According to a poll, the majority of physicians (64%) would wait for molecular testing on a patient metastatic lung adenocarcinoma. Then, upon diagnosis of RET-mutant non–small cell lung cancer, a group of oncologists explained how they would go about treatment for the patient.
GADGEEL: I would like to start off [by asking] what testing you do. Do you always do only tissue-based testing? Do you do plasma-based testing? Do you do both? [Would] any of the participants like to comment as to how they generally approach a patients with stage IV adenocarcinoma?
TEJWANI: At least at Henry Ford [Health System], for stage IV [disease] every patient should get tissue testing, and most of these patients have the tissue-based diagnosis. Liquid biopsies are [used as a] backup. Now we have started in-house testing at Henry Ford, but some [tissue tests are still done by commercial labs, such as FoundationOne CDx] because of insurance coverage.
GADGEEL: I do the same.
NABRINSKY: I agree [about] stage IV [disease. There are] so many actionable mutations now with drugs approved for at least 10 different targeted mutations. Nextgeneration sequencing [NGS] or something similar needs to be done before [initiating] any therapy. [Aside from] stage IV, very seldom do we need urgent therapy. We can wait a few weeks.
GADGEEL: How do you do [NGS testing]? Do you send it locally?
NABRINSKY: It depends on the insurance and the coverage. We usually do 80% [of our NGS testing with] FoundationOne, and some of it goes to Caris [Life Sciences] or other laboratories. But we insist on upfront NGS because it gives you the most detailed information on actionable mutations. If we cannot do [NGS on] solid tumors because of a lack of tissue or insufficient tissue, we do liquid testing, but liquid biopsy-based testing cannot [yet be used to guide anti–PD-L1 therapy].
GADGEEL: That is absolutely true. It’s so encouraging that [NGS testing] has become the standard of care. Even about 2 years ago the testing rates were far lower than they needed to be. I really appreciate that you mentioned that in most patients, not all, we can afford to wait until we get a full molecular panel done, and we may even consider repeat biopsy, if feasible, if the original biopsy tissue is not adequate.
Particularly in the context of this discussion, it is extremely important that [clinicians] are familiar with the type of testing [done at] the lab [the specimen is sent] to. A lot of labs call their tests NGS, but not every NGS test is the same. Particularly when it comes to [testing for genetic] fusions, it is critical that you know what your lab is doing.
[The National Comprehensive Cancer Network guidelines recommend] that if the DNA-based test, which is usually the first test performed, is negative, then a subsequent RNA test be done. Sometimes you can miss [fusions, RET gene rearrangements] on a DNA-based test. [Laboratories] like FoundationOne and Caris clearly do this.
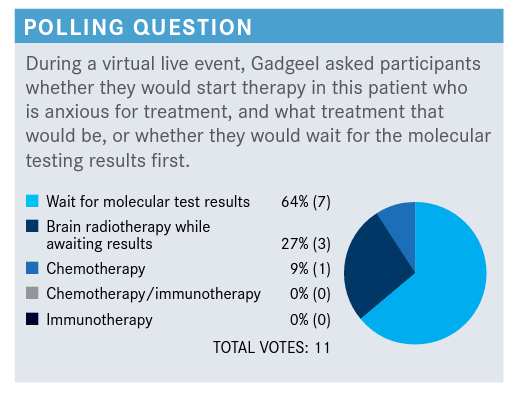
GADGEEL: The majority of folks would wait for molecular results. I would just like to briefly go over this.
I think that even when PD-L1 [expression] is high, it is important to wait for the molecular [assay results] before you start systemic therapy. There are 2 main [reasons] why you generally wait. One, there are data to suggest, at least with some targeted agents, that if you start the patient on immunotherapy and subsequently follow it up with targeted therapy, you may see increased toxicity.1 If [a patient] starts on immunotherapy and subsequently gets a driver genetic alteration, and you want to switch to targeted therapy, there might be a delay.
Second, at least in EGFR or ALK [tumor alterations], and I suspect with other driver genetic alterations, high PD-L1 [expression] could be a result of the downstream signaling effects of the other genes. A downstream effect of EGFR signaling in [EGFR-mutated] tumors may increase PD-L1 expression. [So] PD-L1 expression may not be a result of the presence of immune cells in the tumor microenvironment and may not have the same relevance in patients with driver genetic alterations [as in patients without these alterations].
There are clearly situations where 1 may have to initiate systemic therapy, [such as] if the patient is symptomatic. In such a patient, one would start on chemotherapy alone, see how the patient [does], and wait for the molecular results. Based on the molecular results, one could either add the immunotherapy or switch to targeted therapy.
Finally, when it comes to small asymptomatic brain metastases, the general approach is to wait [before starting] the next systemic therapy to see if early immunotherapy or chemoimmunotherapy [leads to] a systemic response. The location of brain metastases also plays a role [in the choice of therapy].
GADGEEL: About 1% to 2% of nonsquamous NSCLCs are RET rearranged. RET rearrangement occurs in many different tumor types. At present, at least with the drugs we’re going to talk about, there doesn’t appear to be a difference in efficacy, depending upon which [gene] fusion partner is present in the [RET] rearrangement in the tumor. But we need a lot more data to drill down into those issues.
RET mutations have also been observed [in NSCLC]. As we use what I call first-generation RET-specific drugs [pralsetinib (Gavreto) and selpercatinib (Retevmo)], resistance mechanisms [frequently] lead to [subsequent] RET mutations and there are now novel drugs in development that may target [subsequent] RET mutations. Certain tumors may have RET mutations as driver genetic alterations, and in NSCLC usually RET rearrangements are driver genetic alterations, but patients can [subsequently] develop other RET mutations.
GADGEEL: [For those of you] who have managed RET-rearranged NSCLC, can you comment on the patient’s [demographic profile and discuss] brain metastases?
KALAKOTA: I can offer my experience. I’m a hematologist-oncologist and community physician in Chicago. I had a patient, a 49-year-old [woman], nonsmoker, who was found to have metastatic [NSCLC]. She had a RET rearrangement, [which was detected] on molecular profiling. We waited for the molecular profiling results to start treatment.
At that time—this was almost 2 years ago—none of the RET-targeted therapies were commercially available, so I referred her to a tertiary care center locally, [where she was] started on [selpercatinib. At the time of diagnosis] she did not have brain metastases, and a screening MRI did not show any CNS [central nervous system] involvement. She’s been on therapy now for almost 2 years with really good disease control. I have not done routine surveillance for CNS metastases, especially in the absence of systemic progression or new symptoms. Her sites of metastases were the lungs. She had regional lymphadenopathy and liver metastases.
GADGEEL: What therapeutic response did the patient have? Does she still have the disease?
KALAKOTA: [There are] not really major sites of measurable disease anymore, but she did have a lot of [drug] toxicity, which required some dose reductions.
GADGEEL: Let’s say [hypothetically that] this patient had [3 small] brain metastases but was asymptomatic. Would [the presence of brain metastases] have swayed you in terms of how you would approach treatment? Would you have done brain radiation, or would you have waited for the molecular analysis?
KALAKOTA: If she were asymptomatic, I would have waited and started [her on] systemic therapy because there is CNS penetration for these RET inhibitors. If she were symptomatic, I would have referred her for brain radiation.
GADGEEL: Is there a location in the brain that worries you more, and does that sway you in terms of how you would decide [to treat the patient]?
KALAKOTA: What I usually do in these cases is refer the patient to the radiation oncology [department], and we usually take a multidisciplinary approach. [Radiation oncologists] say that their concerns are based on the location and size of the tumor and what the treatment options are. If I think [we are] going to use a targeted therapy that has CNS penetration, [we will] do shortinterval follow-up imaging, but I depend on [the radiation oncologists] for some guidance [on that].
GADGEEL: That’s an excellent point and I’m thankful you brought that up. I will tell you very candidly, about 3 or 4 years ago, when it came to [patients with EGFR- or ALK-mutated tumors] with the activity those [targeted] drugs demonstrate in the brain, I would pretty much just start [patients on anti-EGFR or -ALK therapy] even if [they had] brain metastases.
But over the past 3 years, however small the brain metastases are and even if [the tumor has] a genetic alteration and the patient is going to start on targeted therapy, I will try to get the radiation oncologists involved right from the beginning and comanage [the patient’s care with] them. I think this approach has made a big difference, particularly when it comes to posterior fossa lesions and critical lesions. We sometimes don’t realize the technical aspects of stereotactic radiosurgery, and even if you’re going to start the patient on targeted therapy, I think it is a good practice to get a radiation oncology consultation.
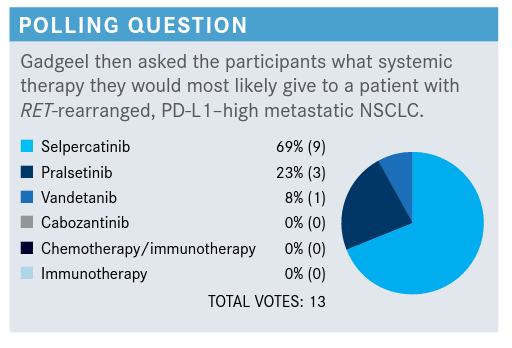
GADGEEL: It looks like there is a hold out with vandetanib, but most folks are going to choose selpercatinib or pralsetinib. I’m very encouraged that despite the fact the patient has a PD-L1–high tumor, nobody chose chemotherapy with or without an immune checkpoint inhibitor or a checkpoint inhibitor alone.

GADGEEL: Can you describe the toxicity [you’ve seen with] selpercatinib?
KALAKOTA: Yes. I had mentioned I sent the patient to a tertiary care center because [at the time selpercatinib] wasn’t commercially available. [The tertiary care center] managed most of the patient’s toxicities [during her initial treatment]. I recently continued her on [selpercatinib] therapy [and she had] liver toxicities. She had significant transaminitis numerous times, [such that we suspended treatment to] wait for the liver profile to improve and did dose reductions. We treated her with steroids at one point and she underwent a liver biopsy showing a drug nonspecific [inflammatory] reaction likely related to drug-mediated liver injury.
She’s now on quite a low dose [of selpercatinib], 40 mg twice a day, and continues to have stable disease. It’s been over a year that she’s been maintained on that dose reduction.
GADGEEL: I’ve treated a few patients [with selpercatinib and], in my experience, [toxicities with this drug are usually not this dramatic].
TEJWANI: You mentioned that 1.8 months was the [median] time to action of [selpercatinib]. If you have a patient with liver metastases on the verge of going into liver failure, would you wait or would you embark on chemotherapy?
GADGEEL: I would still give this drug. [In the LIBRETTO-001 trial (NCT03157128)], the median time to response was 1.8 months because that’s when the first scan was done.2
Generally, scans are done about 6 to 8 weeks into treatment, so that’s where I think the 1.8 months comes in. I don’t want to give the impression that it takes that long for patients to have tumor shrinkage. The tumor shrinkage starts right away. In my experience, a patient notices a difference, just like with anti-EGFR and -ALK drugs, within a week or 2.
TEJWANI: [Access to] the newer drugs [can be delayed] because of co-pays and other insurance issues. From that perspective, if you have [a patient who has a] RET alteration or [is receiving an intravenous PD-1/PD-L1 inhibitor] and you’re waiting for the drug and the patient has multiple liver metastases and liver function tests are abnormal, would you wait until the drug comes [through] or what would you do?
GADGEEL: If there is a [delay] getting the appropriate drug, I think you should always use chemotherapy because that may be your only chance of having the patient live long enough to get the appropriate drug. That is where clinical judgment is going to be very crucial.
In extending what Dr Tejwani said, has anybody had challenges obtaining these targeted oral agents for patients during the COVID-19 pandemic? Or has the pandemic in any way impacted your ability to get molecular testing done?
AHMED: I don’t think [delays in getting these agents] are because of the COVID-19 [pandemic]. As a matter of fact, the use of targeted treatments makes more sense during [this time], as these are oftentimes oral agents that provide an option outside of the infusion room.
GADGEEL: I completely agree. It makes it much easier for the patient. They don’t have to keep coming in every 3 weeks [for intravenous therapy].
BARRAZA: Are there any specific comorbidities that would guide your use [of pralsetinib or selpercatinib]?
GADGEEL: That’s a very good [question]. I will say that [the frequency] of hypertension was somewhat higher with pralsetinib than with selpercatinib [in trial data from LIBRETTO-001 and ARROW (NCT03037385)].2-4
If I have a patient whose blood pressure is not well controlled, I may [choose selpercatinib over pralsetinib]. Outside of that, I’m not certain there was [anything] specific about [comorbidities in the trials]. QTc prolongation was a little more commonly seen with selpercatinib and pneumonitis was [more commonly] seen with pralsetinib, but I’m not sure how much of [the pneumonitis] was necessarily drug related. But these findings are based on very limited datasets and I would [not] use them to decide on 1 [drug] over the other. My sense is that [these drugs are] comparable.
References:
1. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138-1145. doi:10.1016/j.jtho.2018.03.035
2. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusionpositive non–small-cell lung cancer. N Engl J Med. 2020;383(9):813-824. doi:10.1056/NEJMoa2005653
3. Gainor JF, Curigliano G, Kim DW, et al. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(suppl 15):9515. doi:10.1200/JCO.2020.38.15_suppl.9515
4. Gavreto. Prescribing information. Blueprint Medicines Corporation; 2020. Accessed April 8, 2021. https://bit.ly/3kMI8b1