Roundtable Discussion: Ailawadhi Debates Whether MRD Currently Affects Treatment Decisions in Multiple Myeloma
In a rountable Targeted Oncology talk, Sikander Ailawadhi, MD, leads a discussion on whether MRD affects multiple myeloma treatment decisions. He is joined by Ricardo Parrondo, MD Jeffrey Bubis, DO, Mathew Luke, MD, Sushma Simha Nakka, MD, and Thomas Cartwright, MD.
Sikander Ailawadhi, MD

In a rountable Targeted Oncology talk, Sikander Ailawadhi, MD, a professor of hematology and medical oncology at the Mayo Clinic, leads a discussion on whether MRD affects multiple myeloma treatment decisions. He is joined by Ricardo Parrondo, MD Jeffrey Bubis, DO, Mathew Luke, MD, Sushma Simha Nakka, MD, and Thomas Cartwright, MD.

AILAWADHI: How do you discuss the prognosis [for transplant-eligible myeloma] with patients in today’s day and age? How do you define transplant eligibility? And how do you define “young” patients? Many transplant-focused trials used 65 as the cutoff age. Does that mean people at 70, 72, 76, and so on are too old?
PARRONDO: I discuss prognosis with patients based on the data for the use of VRd [bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone] followed by transplant, from the IFM 2009 trial [NCT01191060]1 and an article in the Journal of Clinical Oncology on 10-year followup data of patients treated with VRd.2 About 75% of these patients got a transplant followed by maintenance with lenalidomide. I quote the numbers to talk about prognosis and then transplant eligibility.
In the United States, the age of transplant is getting CyBorD, cyclophosphamide/bortezomib/dexamethasone; KRd, carfilolder. The decision is based not only on the biological age but also on how the patient is doing in terms of functional status and organ function.
I define young patients as those who are younger than 65.
AILAWADHI: At what point do you bring up the discussion about transplant to a new patient with myeloma? Is it at the initial point of diagnosis, when starting treatment, or sometime down the road?
BUBIS: I bring it up at diagnosis.
LUKE: I start at the initial diagnosis. I usually tell them there are many treatments and transplant is 1 [type of treatment]. I tell them [that if they] go into remission, we might send them to a transplant center to get an opinion. Anyone who is 75 years or younger and in good shape is a candidate, as long as the kidney function and [other tests] are good.
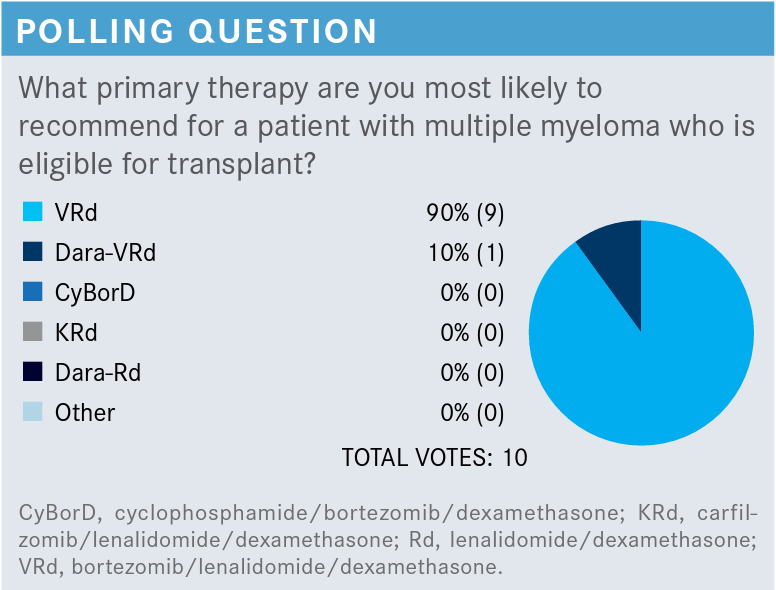
LUKE: I usually start with VRd, especially for a 51-year-old with no neuropathy and no renal function abnormalities. I think VRd is the most effective and is pretty reasonable, so that’s what I start with.
AILAWADHI: Does anyone have an opinion about daraVRd [daratumumab (Darzalex) plus VRD]?
PARRONDO: We have preliminary results from the GRIFFIN trial [NCT02874742].3,4 The addition of daratumumab leads to greater minimal residual disease [MRD] negativity and a sustained complete response,3,5 but I think we need longer follow-up. We know that patients who are MRD negative do better, but we need to see the progression-free survival [PFS] results of the trial.
AILAWADHI: That’s reasonable. We need to wait for the data to mature.

AILAWADHI: Do we think that a patient who is considered transplant eligible—young, fit, etc—is a candidate for doublet therapy in the frontline setting? Does anyone think that there may be a certain category of patients who may benefit from 2 drugs rather than 3?
NAKKA: I use just bortezomib and dexamethasone especially for elderly patients when I think lenalidomide is not going to be tolerated and when the kidney function is really bad. If the kidney function improves, I can always add lenalidomide.

AILAWADHI: You’re right. If a patient has kidney dysfunction, it can be challenging to handle the toxicity from lenalidomide. It is also tricky to change the lenalidomide dose in the middle of the cycle. So it’s reasonable to start with a doublet and then potentially transition to a triplet if needed.
CARTWRIGHT: Probably half to two-thirds. Patients are [receiving diagnoses] somewhat earlier [now], not quite as far advanced. I typically start with 3 drugs. If it’s a person who has renal failure, maybe 2 [to avoid the lenalidomide]. If it’s a patient who is high risk, based on FISH [fluorescence in situ hybridization], possibly 4.
AILAWADHI: I think that’s reasonable; 1 size may not fit all, and it’s reasonable to consider some patient characteristics to decide what their treatment options should be.

AILAWADHI: I wanted to get an idea if you think that depth of response is important in this setting because we talked about MRD negativity.7 Are you doing MRD testing routinely? It is commercially available? Is it reimbursed by insurance? If you do MRD testing routinely, in what setting, and how do you discuss that with the patient?
PARRONDO: At Mayo, we’ve been doing [MRD] after induction, after the transplant, [by] flow cytometry with a sensitivity of 10–5. There are some interesting data from the IFM 2009 trial; patients who were MRD negative before transplant did just as well [as the control group].1 This suggested that patients who are MRD negative perhaps don’t need a transplant, but that remains to be seen in prospective trials.
On the other hand, there aren’t enough data on the effectiveness of starting with a quadruplet regimen or with daratumumab up front; we don’t know yet. There are no data saying at what point the patient will become MRD negative on these regimens. A proportion of patients become MRD negative after VRd induction, and some patients become MRD negative after the transplant. So you might not need a quadruplet regimen to make them MRD negative because some of them become negative with lenalidomide maintenance.
AILAWADHI: It’s interesting you’re bringing that up because we have no way of even suggesting how much the patient would benefit from a transplant. We say that all transplant-eligible patients should receive a transplant. But we don’t know which patients will have a good response and which patients will progress after the transplant. Some people have attempted those kinds of predictive models, but nothing has shown up so far.
LUKE: The MRD—you do it in the bone marrow? I’m not very familiar with it.
AILAWHADHI: Yes, bone marrow. It has been attempted in the blood but not successfully.
CARTWRIGHT: I don’t routinely do [MRD testing]. We have circulating DNA after adjuvant chemotherapy for colon cancer. If it’s positive, it’s not good, but what do you do with the information? So I don’t routinely do it because I don’t see how it is going to change the treatment plan. It’s interesting, but I’m not sure how to use the information.
BUBIS: I agree with that. I think that outside of a research setting, I’m not sure that…more information is not always better. We’re not really sure how to act on it, and it’s not always paid for. So I think that it has clear prognostic implications but no clear therapeutic indications. Until we have data that indicate that you can act on it therapeutically to change the prognosis, I’m not sure that it has a role day to day.
AILAWADHI: You’re right. Right now, if we get an MRD-positive result, we’re not going to switch the treatment plan just to get the patient to be MRD negative. That’s not the goal.
But there are some very kind of neat studies going on where the myeloma world has taken [a page from], let’s say from the CML [chronic myelogenous leukemia] world, or even a little bit I would say from the CLL [chronic lymphocytic leukemia] world, [to see whether there is a role for] adjusting treatment based on MRD.
There is a study that just started through ECOG, where everybody is getting a triplet, and then they’re getting MRD testing. These patients did not intend to do a transplant right away. They are not transplant ineligible, just those who did not plan to do a transplant right away, for whatever reason. So they are checking the MRD [status of these patients] and adding a fourth drug to see whether they can make a difference to the patient’s outcome.
A second large study, happening through SWOG, where posttransplant patients are getting doublet versus single maintenance with DR [daratumumab and lenalidomide] or R [lenalidomide], and then they get time-to-time MRD testing done, and based on MRD testing negativity, de-escalation of treatment is being done, to see whether we can stop maintenance treatment when patients become MRD negative. For how long will they stay MRD negative? When the MRD becomes positive again, does it make sense to add the drugs again, etc? These are large studies with 1000 to 1500 patients being done in the United States. Hopefully, we’ll get a lot more information around that.
I completely agree that MRD positivity/negativity does not provide us with information to make therapeutic decisions at this point. But there are data that show that a deeper response will lead at least to a superior PFS.7 Does that mean that we should pick a regimen that is more likely to give a deeper response? Whether it’s a triplet, quadruplet—that’s a separate issue, but should we be picking treatments that are more likely to give a deeper response to the patient?
BUBIS: I think [we should], within reason. My issue with PFS is that patients don’t ask, “How long until I progress?” They ask, “How long am I going to live?” So the overall survival [OS] is more important. If you have very significant PFS, it would imply that a patient is going to have a better OS. However, it’s not clear. We don’t know at what PFS point you’re going to have [longer OS]. This is particularly difficult in myeloma, given that there are so many different treatment approaches.
You have to consider the wishes of the patient with regard to treatment and potential toxicity. It is more likely that a younger, more fit patient will have a longer life span, based on the fact that there are so many treatment options. You’re probably going to take a different approach with them than with a patient who presents at 70, who might die of another cause, who still has a number of options available from a myeloma perspective.
AILAWADHI: I think that’s reasonable. We don’t fully understand how to use a lot of these drugs for retreatment, and the biggest questions are around monoclonals. We need more studies comparing the benefit of using these drugs concurrently and sequentially. So if you use, let’s say daratumumab, and you’re using it to progression. But on the other hand, we’ve used it for, let’s say 4 cycles, or 2 cycles after transplant, and then maybe maintenance. Is the sequential benefit going to be similar to the single kind of concurrent benefit? Some of those studies have been done in the lymphoma world, for example. But, in myeloma, those data do not exist yet, but I’m happy that some of those studies are going on.
REFERENCES
1. Attal M, Lauwers-Cances V, Hulin C, et al; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
2. Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928-1937. doi:10.1200/JCO.19.02515
3. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
4. Voorhees PM, Rodriguez C, Reeves B, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated efficacy and safety analysis of the safety run-in population of Griffin. Presented at: 2020 American Society of Hematology Annual Meeting and Exposition; December 5-8, 2020; virtual. Abstract 3243.
5. Giri S, Grimshaw A, Bal S, et al. Efficacy of daratumumab in the treatment of multiple myeloma with high-risk cytogenetics: meta-analysis of randomized phase III trials. J Clin Oncol. 2020;38(suppl 15):8540. doi:10.1200/JCO.2020.38.15_suppl.8540
6. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 4.2021. December 10, 2020. Accessed June 20, 2021. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
7. Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. doi:10.1182/blood-2018-06-858613