Roundtable Discussion: Mudad Provides Updates on the Use of Immunotherapy in Non–Small Cell Lung Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Raja Mudad, MD, discussed factors that influence the choice of immunotherapy or chemoimmunotherapy for a patient with metastatic non–small cell lung cancer.
Raja Mudad, MD (Moderator)
Medical Director
Sylvester Comprehensive Cancer Center
University of Miami Health System
Miami, FL

CASE SUMMARY
A man aged 67 years presented with a lump in the left deltoid and no other signs or symptoms. He has a medical history of hypertension and osteoarthritis and is a former smoker with 5 pack-years. An ultrasonography confirmed a 5-cm soft tissue mass in the left deltoid, and a biopsy showed he had squamous cell carcinoma that was thyroid transcription factor 1 negative. A CT scan of the chest, abdomen, and pelvis showed an intramuscular mass in the left deltoid, subcutaneous deposits in the back and thigh, a 5-cm right lower lobe spiculated mass, bilateral bulky mediastinal lymphadenopathy, and a 4-cm right adrenal mass. His brain MRI showed no evidence of central nervous system metastasis and was ultimately considered stage T2aN3M1b.
An immunohistochemistry showed a PD-L1 tumor proportion score of 10% (22C3 antibody assay) and next-generation sequencing was negative for aberrations in EGFR, ROS1, BRAF, ALK, RET, MET, HER2, NTRK, and KRAS. He was microsatellite stable and had a tumor mutational burden of 6 mut/Mb. A decision was made to treat the patient with a platinum taxane, immune checkpoint inhibitor (ICI) regimen.
DISCUSSION QUESTIONS
- What factors support a decision to use an ICI plus chemotherapy instead of ICI monotherapy in this patient with metastatic non–small cell lung cancer (mNSCLC) and a PD-L1 expression level of 10%?
- Which key factors influence your decision-making for patients with mNSCLC with a PD-L1 combined positive score of 1% to 49%?
- Use of ICI plus chemotherapy vs ICI doublet plus chemotherapy vs ICI monotherapy?
- Selection of ICI agent?
- Do you ever consider combination ICIs?
MUDAD: I am convinced that a single agent [for patients with a combined positive score] of under 50% is not the way to go. I think [most] oncologists would treat these patients with a combination of chemoimmunotherapy. A question that comes up is what immunotherapy…you use. What’s the most common regimen you use? We have several combinations approved.
NGUYEN: For me, [that agent is] pembrolizumab [Keytruda].
WERTHEIM: For this patient, I might use combined [ipilimumab (Yervoy) plus nivolumab (Opdivo)] with chemotherapy because of his squamous cell [disease] and low PD-L1 expression.
HURTADO: Yes, I agree with the previous comment. Because of the disease burden, I probably would give a couple of cycles of chemotherapy with the ipilimumab plus nivolumab. I think the patient will do very well with that.
MUDAD: I’m a big fan of dual immunotherapy. I think dual immunotherapy is a very good combination. [Most] patients with a PD-L1 expression level of 1% to 49% are probably treated with a single agent, but dual-agent immunotherapy plus chemotherapy…is powerful.
To your point, Dr Wertheim, I think the lower the PD-L1 expression, the more I feel that I [must] use more than just a single agent, because although we group these patients as “expression level of 1% to 49%,” clearly a patient who has an expression level of 2% is not the same as one who has an expression level of 48%. There’s a proportional response [with respect] to how high the PD-L1 expression is. So, although we group them together, for patients with lower PD-L1 expression, I am more likely to use dual immunotherapy. I would use single immunotherapy plus chemotherapy in a patient whose expression level is closer to 50%.
We talked about tumor burden as one of the things to influence your decision…. Dual immunotherapy…is more aggressive, so for a patient with a higher tumor burden I am more likely to use that. [You can also] consider cemiplimab-rwlc [Libtayo] plus chemotherapy, which was recently approved.1
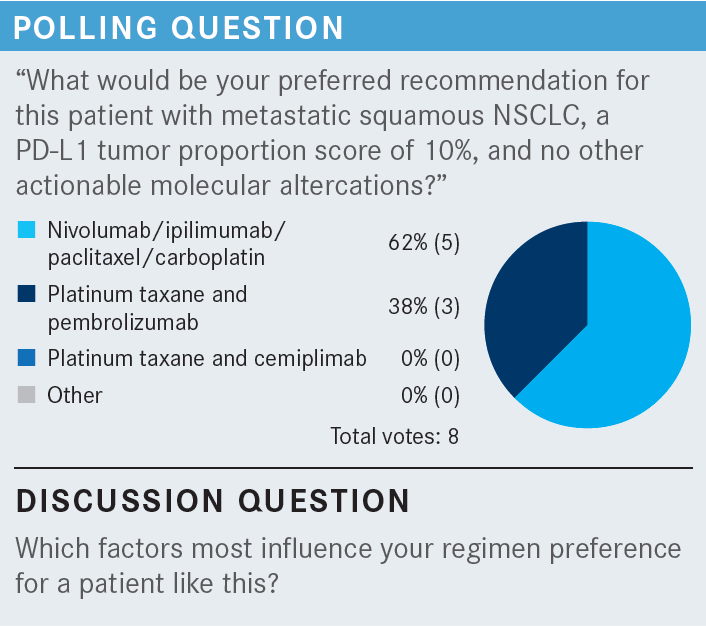
MUDAD: The discussion here is about what influences what we use. There has not been any comparison of dual immunotherapy vs single immunotherapy with chemotherapy. I don’t think we will ever have that type of trial, but physicians clearly believe it’s efficacious to use dual immunotherapy.
For those who have used the combination, did you have any increased toxicity from the combination of immunotherapy? I know a lot of physicians are worried when they see ipilimumab, especially if they’ve used ipilimumab for something other than lung cancer. In melanoma trials, they’ve used higher doses—and toxicities, initially, were high. As you know, in lung cancer, when we use this combination, ipilimumab is used at 1 mg/kg, and it’s used every other cycle, not every cycle. It’s every 6 weeks. The median number of doses in that trial [CheckMate 9LA (NCT03215706)] was 4, so [most] patients were receiving 4 doses of ipilimumab at the very low dosage of 1 mg/kg every 6 weeks. The data showed that there was usually not increased toxicity when compared to single-agent nivolumab, when this combination was used at this dosage.2
NGUYEN: I regret using chemotherapy plus ipilimumab/ nivolumab in a patient in whom, in hindsight, I shouldn’t have. I should have just used combined immunotherapy by itself. I think the toxicity is in the chemotherapy.
MUDAD: We also know that the combination of ipilimumab/ nivolumab alone is also approved for this patient population.3 I see, sometimes, the advantage of using this regimen vs, for example, the combination of carboplatin, pembrolizumab, and pemetrexed [Alimta, carbo/pem/pem], because with carbo/pem/pem, [patients receive] chemotherapy plus immunotherapy, 4 cycles, and then you do maintenance with pemetrexed. In the CheckMate 9LA trial of ipilimumab/ nivolumab, there were only 2 cycles of chemotherapy and there was no maintenance pemetrexed.
They only maintained the patients on ipilimumab/ nivolumab, and the ipilimumab was [given] every other cycle [From the Data2]. If patients don’t want chemotherapy, and if they are not eligible to have only ipilimumab/nivolumab because their PD-L1 expression is less than 1%, a way to [convince] them is [to point out that they would] only get 2 cycles of chemotherapy plus ipilimumab/nivolumab, not 4 cycles, and they would not be getting maintenance pemetrexed but rather maintenance ipilimumab/nivolumab or nivolumab alone.
Burden of disease is something that would make me want to use dual immunotherapy. Site of metastases [is another consideration]. A lot of the early immunotherapy trials excluded patients with brain metastases. The later trials, especially with single-agent cemiplimab, allowed patients with brain metastases as long as the patients were treated and stable. The response in the brain, particularly with cemiplimab, was impressive.4 So, brain metastases may sway me to consider using cemiplimab. In general, squamous disease is considered more difficult to treat…[and] I would probably use more aggressive therapy. The dosing schedule for ipilimumab is every other cycle; but with electronic medical records, the regimen is built in, so we don’t have to worry about when to skip the ipilimumab or add the ipilimumab.
Any patient with EGFR or ALK mutations…[must] be treated with a targeted agent, with the exception of KRAS[-targeted agents] in the first line, which are still not approved, so it would be your choice of treatment followed in the second line by a KRAS inhibitor.

NGUYEN: The PD-L1–negative subgroup didn’t seem to benefit as much.4
MUDAD: Yes, the HR for overall survival among patients with PD-L1 expression of less than 1% [was 1.01; 95% CI, 0.63-1.60]. This could have been because of patient numbers, although [the PD-L1 expression subgroups had similar numbers of patients].4 One can argue that patients with expression levels of less than 1% may not do as well. In those patients, I almost always use dual immunotherapy. I don’t see that there is much role for this combination in [this population]. That certainly can be one point to conclude, or [at least] to discuss, from [these data].
Do brain metastases have any influence over what agents you use?
CALDERA: It hasn’t been a decisive factor until now, with this new suggestion that cemiplimab could work. I believe that on squamous [disease], cemiplimab would make more sense than pembrolizumab for some of these patients. I haven’t used the doublets yet, but I think that the way the field is going, pembrolizumab is not going to be used as much for the squamous histologies.
MUDAD: I agree. I also think that because of the history of cemiplimab being active in [patients with] squamous disease in other organs, such as skin, we probably will favor [cemiplimab]. I favor it a little more in brain metastases because there are data out there.4 I don’t have any problem with adverse events [AEs]. I think any of the combinations of chemotherapy plus a single immunotherapy are pretty much the same in terms of AEs. I don’t see any difference in picking one or the other because of AEs.
Do you have the liberty to pick whatever [agent] you want in your practice, or do you have guidelines that tell you what you should or should not use?
WERTHEIM: I’m of the opinion that all the PD-1– and PD-L1–[targeting agents] are quite similar in lung cancer and in other diseases as well. In private practice, it’s going to be tough not to use this, because, financially, it makes better sense to use it until pembrolizumab becomes generic.
MUDAD: OK. So, there is an incentive by the company to use cemiplimab?
WERTHEIM: Yes.
MUDAD: These companies ask, “Well, what do you think? How can we break into the market?” When you’re the third one to come on a market where physicians have used other drugs for a long time, it’s hard to break in, especially if your drug doesn’t have any big difference compared with the others. It also boils down to which one is cheaper, and I think it makes sense that if it’s cheaper on the system, cheaper for the practice, [and] more beneficial besides, those certainly are reasons to use it.
MUDAD: Do you have any reactions to the approval of this regimen?5 Sometimes we say, “Do we need yet another combination of drugs?” Certainly, the market is now saturated. The bottom line is for us to decide how we use this agent vs another, and each one of us will have to find something in the data that we find more convincing or a subgroup of patients who may benefit more from this agent vs another….
We probably would use dual immunotherapy vs single immunotherapy for squamous disease. Tumor burden [is another consideration] and in this group [of patients], if a patient has an STK11 or a KRAS [mutation], I probably would want to try this regimen. Has anybody [here] used it?
NGUYEN: Overall, compared to the ipilimumab/nivolumab regimen, is the CTLA-4 [inhibitor] used more in this regimen or the other regimen?
MUDAD: The CTLA-4 [inhibitor] is used more in the ipilimumab/nivolumab regimen; it’s built in to continue, so some patients have continued every other cycle on ipilimumab until toxicity.2 The [POSEIDON regimen] only calls for 5 [cycles].6
CTLA-4 [inhibition] is thought to drive the T lymphocytes from the central compartment to the tumor. So, if you have a tumor that doesn’t respond to immunotherapy, meaning it doesn’t have enough tumor-infiltrating lymphocytes, and you use a PD-1 or a PD-L1 inhibitor, it isn’t going to do anything, because the tumor and the T cells aren’t seeing each other. But, if you drive T cells to the tumor, the combination makes sense. The rationale [in the POSEIDON study (NCT03164616)] was to drive all the T cells early, so they gave 5 cycles of tremelimumab. They didn’t skip cycles.6 The other group did it gradually, every other cycle.2 I don’t think there was any scientific explanation for either [method vs the other].
Are you open to trying it? I’m very interested in trying it, especially if the patient has an STK11, KEAP1, or KRAS mutation. Do you ever do anything different, Dr Caldera, if you have [patients whose disease has] these mutations?
CALDERA: Not really, no.
MUDAD: I think this may be a peek at the future, that we will start identifying subgroups that may benefit from one regimen vs another. The subgroup that is always a challenge is those patients with PD-L1 expression of less than 1%.
I think those patients deserve dual immunotherapy. Most of the single-agent immunotherapy trials don’t do very well with that population. In that group, I use ipilimumab/nivolumab plus chemotherapy, or I will be trying this regimen. It would be ideal to use ipilimumab/nivolumab [alone], but it’s not approved for use in that group.3 Only ipilimumab/nivolumab plus chemotherapy is approved for that group of patients.7
HURTADO: Are you concerned that there have been many trials with durvalumab and tremelimumab and they have failed, especially in other tumors? And it seems to me that they did the subgroup analysis just to show some sort of difference, but I’m not sure, if you did this in the other trials, if you would see some similarity with what they found here with…KRAS.6,8
MUDAD: That’s a reasonable question. I don’t know what to say about the durvalumab and tremelimumab in other trials. You’re right, this is the first time we have a positive trial. Whether it was the design of the trials, [I don’t know]. [Regarding] the subgroup analysis, I agree. If it’s confirmed in a prospective trial, I think it will be a bombshell. I’ll try it; we’ll see and we’ll get experience with it. I’ve never used durvalumab or tremelimumab before, but I’m always open to trying something new.
REFERENCES
1. FDA D.I.S.C.O. Burst Edition: FDA approval of Libtayo (cemiplimab-rwlc) in combination with platinum-based chemotherapy for non–small cell lung cancer. FDA. Updated December 5, 2022. Accessed March 14, 2023. https://bit.ly/40LyMR3
2. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi:10.1016/j.esmoop.2021.100273
3. FDA approves nivolumab plus ipilimumab for first-line mNSCLC (PD-L1 tumor expression ≥ 1%). FDA. Updated May 15, 2020. Accessed March 14, 2023. https://bit.ly/40UOAR1
4. Gogishvili M, Melkadze T, Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non–small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28(11):2374-2380. doi:10.1038/ s41591-022-01977-y
5. FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non–small cell lung cancer. FDA. Updated November 18, 2022. Accessed March 14, 2023. https://bit.ly/40Drizo
6. Johnson ML, Cho BC, Luft A, et al; POSEIDON Investigators. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41(6):1213-1227. doi:10.1200/JCO.22.00975
7. FDA approves nivolumab plus ipilimumab and chemotherapy for first-line treatment of metastatic NSCLC. FDA. Updated May 27, 2020. Accessed March 15, 2023. https://bit.ly/3U56Ftg
8. Johnson M, Cho BC, Luft A, et al. Durvalumab (D) ± tremelimumab (T) + chemotherapy (CT) in 1L metastatic (m) NSCLC: overall survival (OS) update from POSEIDON after median follow-up (mFU) of approximately 4 years (y). Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/annonc/assonc1089
