Garmezy Reviews Data for Frontline Therapy in Advanced RCC
During a Targeted Oncology™ Case-Based Roundtable™ event, Benjamin Garmezy, MD, reviewed updated results of trial of frontline combination therapies for patients with advanced renal cell carcinoma.
Benjamin Garmezy, MD
Assistant Director of Genitourinary Research
Sarah Cannon Research Institute at Tennessee Oncology
Nashville, TN

Targeted Oncology: What data support the use of cabozantinib (Cabometyx) plus nivolumab (Opdivo) as a first-line regimen in advanced RCC?
GARMEZY: The CheckMate 9ER study [NCT03141177] is what got [cabozantinib and nivolumab] FDA approved in 2021.1 The dose was different from the single-agent dose, which is typically started at 60 mg daily. Nivolumab plus cabozantinib at 40 mg daily was compared with sunitinib [Sutent], which is dosed 4 weeks on, 2 weeks off, which is different from continual cabozantinib dosing. Patients had RCC with a clear cell component. Patients in any IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] risk group were allowed to enroll. The primary end point was progression-free survival [PFS], and the key secondary end points were overall survival [OS], objective response rate [ORR], and safety.2
The median PFS was 16.6 months for cabozantinib and nivolumab and 8.3 months for sunitinib. The HR was 0.56 [95% CI, 0.46-0.68], which is a low HR at the 33-month median follow-up. These are good data. At 24 months, the PFS rate was nearly 40% of patients on cabozantinib and nivolumab compared with 21% of patients on sunitinib. That’s a large jump. That’s about double the [percentage of] patients, so I think those are striking data, and [those] are updated from data presented in 2022.3
From the 33-month follow-up data, the OS rate at 2 years was 70.3% vs 60.3% for sunitinib, so that’s a sizable difference as well. The median OS was 37.7 months vs 34.3 months. The HR is still there at 0.70 [95% CI, 0.55- 0.90], so a 30% difference. The median OS isn’t that numerically different, but based on the Kaplan-Meier curves and the separations through 3 years, I think that’s impressive and clinically meaningful for our patients as far as giving them a doublet therapy vs sunitinib single-agent TKI [tyrosine kinase inhibitor].2
What were the subgorup analysis and response rate results in CheckMate 9ER?
The subgroup analysis is critical, although the trials aren’t powered to have a strong statistical significance to look at these single subgroups. What you want to see in a good doublet that you’re going to use in many patients is a trend favoring nivolumab and cabozantinib compared with the sunitinib control. There’s a little bit of difference [among] poor, intermediate, and favorable groups in the IMDC prognostic risk. They all benefit from nivolumab plus cabozantinib, but it does seem the patients with poor-risk [disease] did much better. Across all subgroups, patients derived benefit from the doublet.2
The response rates are also critical, especially because many [physicians think] it’s important for a patient who needs a quick response to give them something that’s going to cause that response, because you may only get 1 chance at the front line. The ORR with nivolumab plus cabozantinib was 55.7% vs 28.4% [with] sunitinib. That’s a [difference] of 27.3%, which is striking, and it’s basically double when you compare those response rates. The primary progressive disease rate was 6.2% on the doublet vs 13.7% on sunitinib. The median duration of response is also striking. Not only do they respond, but they respond for longer. On sunitinib that’s 15.1 months, and on nivolumab plus cabozantinib it is 23.1 months.3 So that’s striking and clinically meaningful.
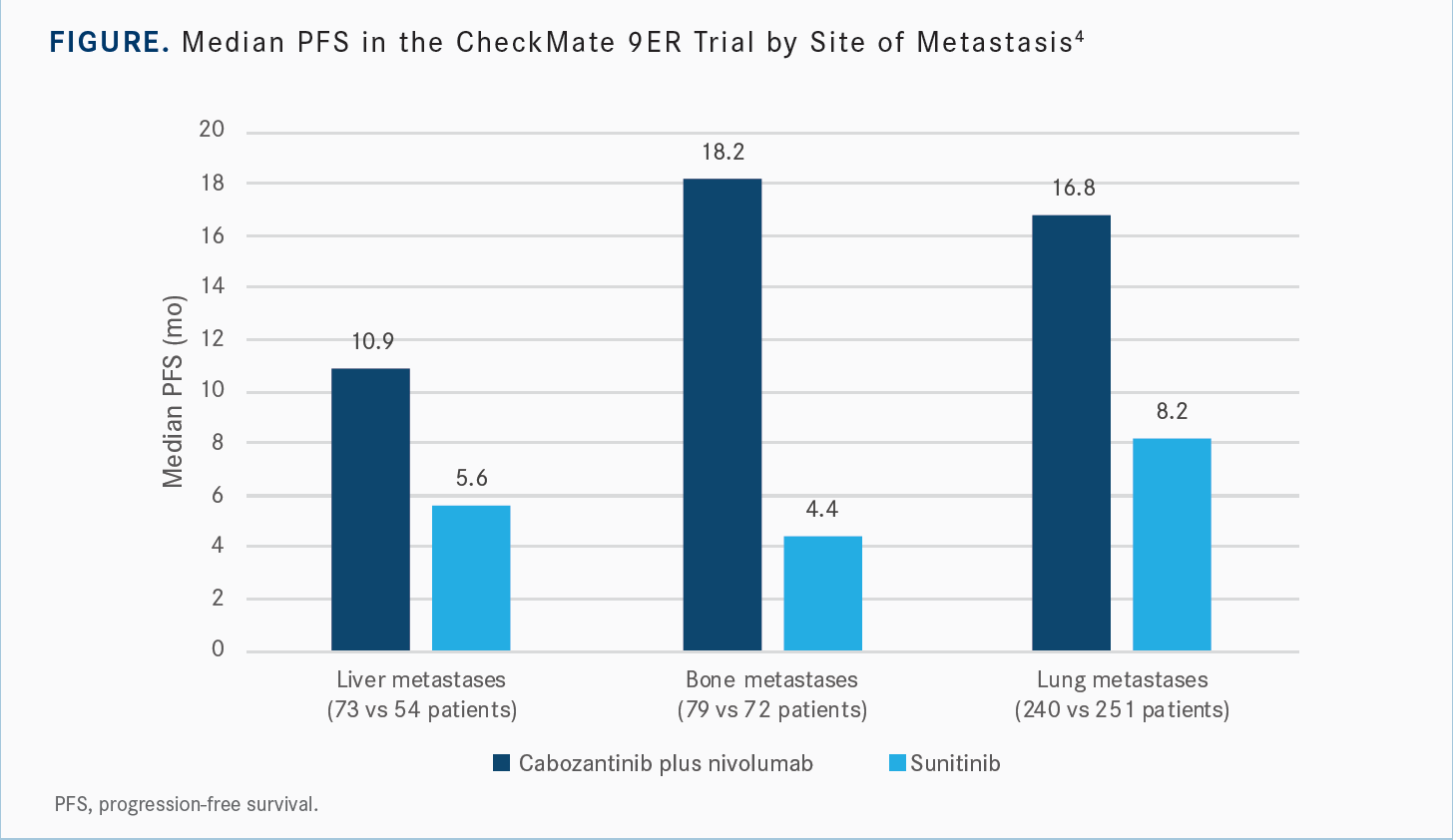
[It is thought that] cabozantinib could perhaps be better for patients with bone metastases because of the data. I also used to do research on RCC bone metastases and felt that cabozantinib was a good agent in those patients based on some other work that’s been done in that area. So I’ll second that, but the key is you’re seeing benefits in all types of disease for both PFS and OS compared with sunitinib.
There’s not a specific organ that this doublet doesn’t work better [for] than the single-agent TKI. The Kaplan-Meier curve [for PFS with] bone metastases is even more striking than that of the liver and the lung [Figure4]…. There are some data suggesting that other TKIs also perform well [for] solid tumors as far as where the disease burden is. So this may not be only for cabozantinib plus nivolumab. But it’s important to see, and I’m glad they presented [those] data.
The toxicity is predictable. I think [oncologists] are familiar with at least giving TKIs and IO [immuno-oncology]. So 10% to 11% of patients had to discontinue nivolumab, 9.1% discontinued cabozantinib, and 7.5% discontinued the combination. For sunitinib, about 10% discontinued. It’s not that different when you look at both arms, and toxicity was as expected for the agents. [Patients experienced] diarrhea, hand-foot syndrome, hypertension, fatigue, hypothyroidism, and all the things we expect to see when we give these drugs. It is a well-tolerated regimen, and when you compare it with sunitinib, they look equivalent even with the nivolumab [added]. There [are higher] liver enzyme [levels] when you give the combination doublet compared with sunitinib, so I think that’s important but something we monitor routinely.3
How was pembrolizumab (Keytruda) plus axitinib (Inlyta) investigated as a first-line regimen in RCC?
The KEYNOTE-426 study [NCT02853331] compared pembrolizumab plus axitinib with sunitinib. The axitinib dose is 5 mg twice a day. The study had dual primary end points of OS and PFS. But otherwise, the trial design was like the CheckMate 9ER study. It was in the front line and in any IMDC risk group, which was later stratified.5
The OS HR was close to cabozantinib plus nivolumab at 0.73 [95% CI, 0.60-0.88; P < .001]. At 18 months, it was 81% vs 72% for sunitinib. At 36 months, it was 63% vs 54% for sunitinib, so it had a similar delta [to CheckMate 9ER] if we’re trying to compare [indirectly between separate trials]. The median OS [at 42.8-month median follow-up] was 45.7 months with axitinib/pembrolizumab vs 40.1 months for sunitinib.
The subgroup analysis mostly suggested that the doublet is better than sunitinib alone. For IMDC risk score, the favorable-risk group did not see much benefit with the doublet. So there may be less of a difference when you give the doublet vs the single agent. This trial was not powered to ask that question or answer that question, but it’s hypothesis generating nonetheless.
The PFS had an HR of 0.68 [95% CI, 0.58-0.80; P < .0001]. At 18 months, the PFS rate is 48% for the doublet vs 35% for sunitinib, and at 36 months it’s 29% for the doublet vs 15% for sunitinib. The median PFS [at a 42.8- month median follow-up] was 15.7 months with the doublet vs 11.1 months for sunitinib.
The [percentage] of patients with a confirmed ORR, including CRs [complete responses] and PRs [partial responses], was 60.4% vs 39.6% for sunitinib. There’s more of a response with sunitinib in this trial, but overall, it’s relatively similar and comparable. The median duration of response was 23.6 months with axitinib/pembrolizumab vs 15.3 months for sunitinib, so [it’s] comparable with what we saw [in CheckMate 9ER].
Safety is also similar. Hypertension is a little more common, and that’s perhaps because axitinib is more VEGF selective than cabozantinib. Among the TKIs, you can group axitinib and tivozanib [Fotivda] as more VEGF-selective TKIs. Cabozantinib and lenvatinib [Lenvima], on the other hand…have more targets. The hypertension comes from the VEGF targeting, so we get a little more of that with axitinib. Perhaps some of the other adverse event [AE] profile is changed as well, but overall, they are similar, and we’re familiar with these classes of medications.
Discontinuation occurred in approximately 28% for pembrolizumab, about 20% for axitinib, and about 7% for the combination. Sunitinib was close to the CheckMate 9ER trial at 12%. It’s important that there [were] more discontinuation and dose interruptions, and perhaps that was due to tolerability or other issues on the trial.
What data support the use of lenvatinib plus pembrolizumab as a first-line regimen in patients with advanced RCC?
The CLEAR study [NCT02811861] used the most recent doublet on the block. This was lenvatinib at 20 mg daily plus pembrolizumab. That’s a very high dose [for lenvatinib]. If we compare it [with] cabozantinib at 40 mg daily and axitinib at 5 mg twice daily, we would probably agree that lenvatinib is the strongest. The primary end point was PFS, which was a difference in the trial design. Patients were [randomly assigned] 1:1:1 to receive sunitinib, lenvatinib plus everolimus, or lenvatinib plus pembrolizumab. The lenvatinib was at 18 mg and everolimus at 5 mg in the everolimus arm.6 What made its way forward was lenvatinib plus pembrolizumab, but [lenvatinib plus everolimus] does remain an important subsequent-line regimen in RCC for patients who are [experiencing progression].
The median PFS for lenvatinib plus pembrolizumab [at median follow-up of 33.7 months] was 23.3 months, and for sunitinib it was 9.2 months. For the IMDC risk groups, the poor-risk category had the smallest HR of 0.30, favorable-risk [category] at 0.47, and intermediate-risk [category] falling in the middle at [0.41].7
All subgroups favored the doublet compared with sunitinib. But we don’t see that change in the IMDC risk groups. It’s all flat with favorable-, intermediate-, and poor-risk categories. There are relatively new data presented at ESMO [European Society for Medical Oncology Congress 2021] looking at whether the [main] site of disease matters.8 Patients with bone and liver metastases were still responding on the doublet similarly, so that didn’t change. The sarcomatoid component, which I haven’t talked about yet [in other studies], also did similarly across subgroups.
For OS, the [Kaplan-Meier] curves start to converge after about 3 years, but we’re still waiting to get more data. We don’t have a median OS for lenvatinib plus pembrolizumab because patients still haven’t crossed that part of the curve yet. At 37.7 months, the median OS is significantly higher for pembrolizumab and lenvatinib compared with sunitinib. The IMDC risk curve is interesting; the HR, unlike for PFS, does show that swing between poor risk and favorable risk, with the HR for poor risk being 0.39 and favorable risk being 1.22.7 That’s pretty interesting, and we’ll just have to see how this evolves as more data get presented.
The OS rate at 36 months for patients who completed the 2 years of pembrolizumab was 94.5%, which is striking; [however,] it’s a cherry-picked population. If they make it that far and finish the pembrolizumab and they’re on lenvatinib monotherapy, most of them are going to be alive at 3 years. The survival probability falls off after that discontinuation at the 3-year mark, so it’s something to keep in mind, and something that we’ll track going forward.
[It may be thought that] ipilimumab [Yervoy] plus nivolumab has the highest CR rate [in the CheckMate 214 trial (NCT02231749) results]. That’s not necessarily true.
It has the longest durability of response data, so we know that the CR can be durable.9 The doublet CR rate [for lenvatinib/pembrolizumab] was 17.2%. With cabozantinib plus nivolumab, it was [approximately] 10%, which was comparable with the initial data presented from ipilimumab plus nivolumab. So these are important and striking data.
The primary progressive disease rate was only 5.4% on the doublet, very comparable with cabozantinib plus nivolumab. [With] sunitinib, it’s a bit higher at 14%. The median duration of response was similar, but this time it’s a bit higher in both arms at 26 months with lenvatinib/ pembrolizumab vs 14.7 months for sunitinib.7
The AEs are similar [to the other trials] with more grade 3 AEs. With this combination, because most likely the lenvatinib dose was higher, more discontinuation was expected. So 25.6% of patients discontinued lenvatinib [because of] AEs, pembrolizumab was 28.7% of patients, and those who discontinued both were about 13.4% of patients. And sunitinib was at 14.4% of patients, which is slightly higher than that of the other 2 studies as far as comparing control arms.
Dose reduction [was] about 70% for the doublet arm, so most patients did not tolerate the combination with lenvatinib at 20 mg, which is not surprising for anyone who’s given these drugs. Nearly 80% required a treatment interruption at some point with this doublet.6 So this was a higher dose of TKI with a higher response but also higher toxicity.
How do these frontline trials for advanced RCC compare?
The ORR rate was a bit higher in lenvatinib plus pembrolizumab at 71% compared with 56% with cabozantinib plus nivolumab and 60% with axitinib plus pembrolizumab. CR rates were higher with lenvatinib plus pembrolizumab at 17% compared with both cabozantinib plus nivolumab at 12% and axitinib plus pembrolizumab at 10%.
So patients are getting CRs on these TKI [plus immunotherapy] doublets. The primary progressive disease is something we’ve been talking about a lot. It seems to be the lowest [in] cabozantinib plus nivolumab and lenvatinib plus pembrolizumab.2,5,6
The treatment-related AEs of any grade are basically above 90% for all these.2,5,6,10 So [nearly all patients] are going to have some toxicity. For grade 3 or greater AEs, lenvatinib plus pembrolizumab was at 72% vs 59% for control, cabozantinib plus nivolumab was at 65% vs 54% for control, pembrolizumab plus axitinib was at 68% vs 64% for control, and ipilimumab plus nivolumab was at 48% vs 64% for control.
There were fewer grade 3 AEs with ipilimumab plus nivolumab at the 40% to 50% mark. The discontinuation is highest with ipilimumab plus nivolumab and lenvatinib plus pembrolizumab compared with the other agents as well as axitinib, which was near 20%. Cabozantinib plus nivolumab had the lowest discontinuation when compared across trials, which isn’t always fair [to compare], at around 7%.
Corticosteroid use, which wasn’t reported for pembrolizumab plus lenvatinib, was similar across trials from the data that we do have. There are health-related quality-of-life [HRQOL] data that are emerging. From the KEYNOTE-426 and CLEAR studies, the HRQOL data aren’t worse or better than [those for] sunitinib.11,12 In the CheckMate 9ER study [data], there are statistically significant [differences] in HRQOL outcome reports.13
It’s always challenging to do these types of surveys [with] patients receiving sunitinib because it depends on whether you survey them at that 4-week mark when they’re on the drug or at that 6-week mark when they’re off the drug, because they have had a treatment holiday built in where they’re going to feel better vs when they’re going to feel worse. There were differences on how the HRQOL data collection was done [in] these trials, so I don’t want to draw too much [from the comparison]. But it is nice to see that in the CheckMate 9ER study [results], the doublet is not only better than sunitinib, but it’s also potentially better tolerated and with a statistically significant difference.13
References
1. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed March 14, 2023. https://bit.ly/3LmtuXl
2. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
3. Thomas P, Choueiri TC, Mauricio B, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. Abstract presented at: 2022 American Society of Clinical Oncology Genitourinary Cancers Symposium; February 19, 2022; San Francisco, CA. Abstract 350.
4. Apolo AB, Powles T, Burotto M, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. J Clin Oncol. 2021;39(suppl 15):4553. doi:10.1200/JCO.2021.39.15_suppl.4553
5. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
6. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
7. Porta CG, Eto M, Motzer R, et al. Updated efficacy of lenvatinib (LEN)+pembrolizumab (PEMBRO) vs sunitinib (SUN) in patients (pts) with advanced renal cell carcinoma (aRCC) in the CLEAR study. Ann Oncol. 2022;33(suppl 7):S660-S680. doi:10.1016/annonc/annonc1072
8. Choueiri TK, Eto M, Kopyltsov E, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Ann Oncol. 2021;32(suppl 5):S678-S724. doi:10.1016/annonc/annonc675
9. Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085-2097. doi:10.1002/cncr.34180
10. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi:10.1136/esmoopen-2020-001079
11. Bedke J, Rini BI, Plimack ER, et al. Health-related quality of life analysis from KEYNOTE-426: pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. Eur Urol. 2022;82(4):427-439. doi:10.1016/j.eururo.2022.06.009
12. Motzer R, Porta C, Alekseev B, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23(6):768-780. doi:10.1016/S1470-2045(22)00212-1
13. Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. doi:10.1016/S1470-2045(21)00693-8

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen