Komrokji Discusses Therapeutic Options in Patients With Intermediate-Risk Myelofibrosis
During a Targeted Oncology™ Case-Based Roundtable™ event, Rami Komrokji, MD, discussed the use of JAK inhibitors for treatment of patients with primary myelofibrosis.
Rami Komrokji, MD
Vice Chair, Department of Malignant Hematology
Moffitt Cancer Center
Tampa, FL

Targeted Oncology: Can you discuss the National Comprehensive Cancer Network (NCCN) guidelines for myeloproliferative neoplasms (MPNs)?
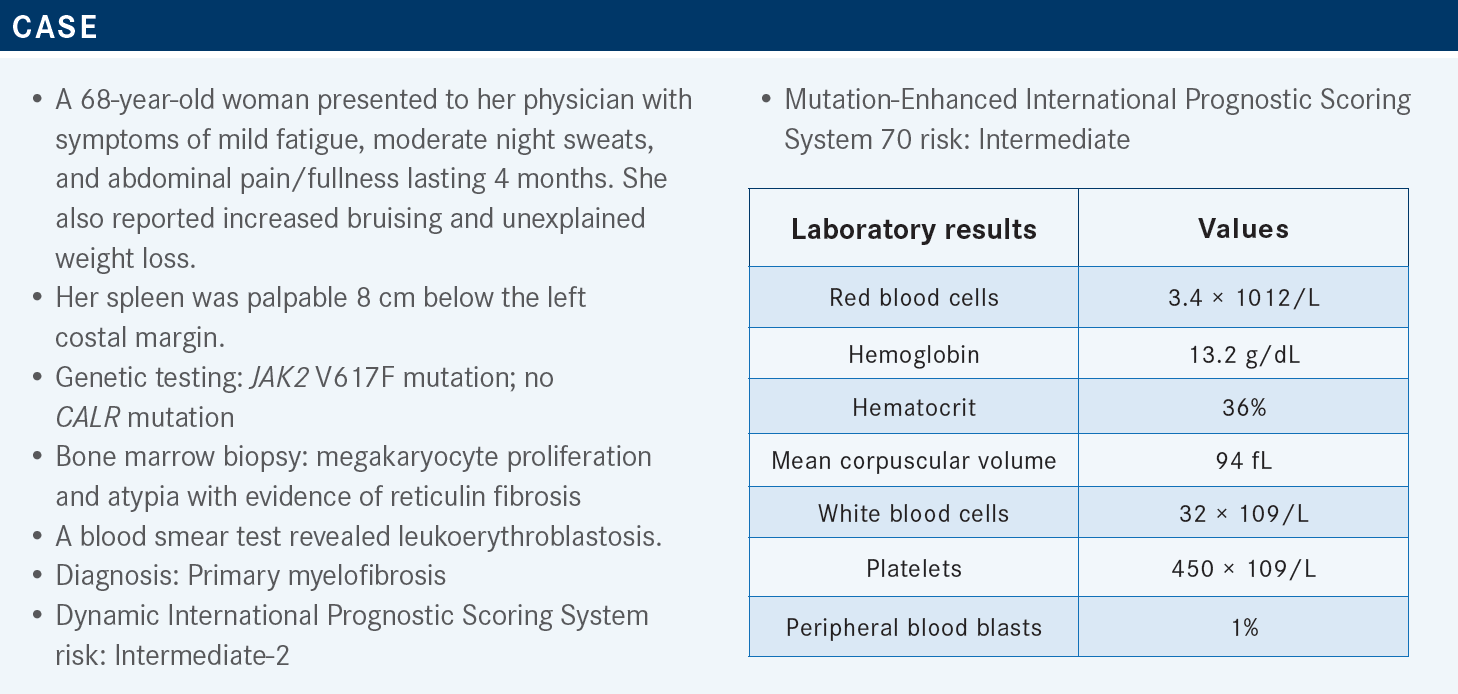
KOMROKJI: The revised NCCN guidelines are based on the 3 JAK2 inhibitors available.1 If patients’ disease is intermediate or higher risk and they have low platelet [count] below 50 × 109/L, if they are transplant candidates, they receive a transplant. But most of the time, we start [with] JAK2 inhibitors and then proceed to transplant. There are data that if patients were responding to JAK2 inhibitors and [underwent] transplant, they had better outcomes [due to] improved performance and reduction in spleen [size].
If the platelet [count is] below 50 × 109/L, the standard is pacritinib [Vonjo] in our center. If the platelet [count is] above 50 × 109/L, per the NCCN, ruxolitinib [Jakafi] or fedratinib [Inrebic] are both listed. Most [individuals] have used ruxolitinib for a longer time and are more comfortable with that; [it] could be used as a bridge for transplant.
What are some other factors you think about when using these 3 JAK2 inhibitors?
There are some slight differences among them. By the label, ruxolitinib is approved for patients with platelet [count of] 50 × 109/L and above, pacritinib for platelet [count] below 50 × 109/L, and fedratinib does not have a cutoff on the count approval.
Ruxolitinib is a JAK1 and JAK2 [inhibitor]. Fedratinib is a bit more specific for JAK2. It does have some FLT3 activity. Pacritinib does not have much JAK1 activity; it is more JAK2. It also affects IRAK1, which is a part of the myddosome pathway. So it is another inflammatory pathway, and it does have some FLT3 activity.
The more we have JAK1 activity, there are probably more related cytopenias, and [it’s] more likely you are going to get some withdrawal if you stop. We see a lot of withdrawal with ruxolitinib. If you have FLT3 activity, both fedratinib and pacritinib have a little more gastrointestinal toxicity. Use the guidelines on the package insert for the ruxolitinib starting dose and adjust accordingly.2
Typically, it is week 8 where we see the nadir of counts for both the anemia and the platelets. Neutropenia is not that common with ruxolitinib, but there are different schools [of thought]. The University of Texas MD Anderson [Cancer Center in Houston] starts from the highest dose, and their argument is that those milestones at 3 or 6 months correlate with response. Sometimes I will start at a dose level below the package insert if I am not sure, especially if the counts are borderline, with the notion that I would escalate the dose faster if patients are not getting profound cytopenia.
Can you go over the response data for ruxolitinib from the COMFORT trials?
Ruxolitinib is the oldest [JAK2 therapy available]. It has been approved for a while based on 2 trials, COMFORT-I [NCT00952289] and COMFORT-II [NCT00934544].3,4 One was done in the United States, and the control arm was placebo. COMFORT-II was done in Europe, and the control arm was best available therapy. The gold standards were those end points: spleen response by MRI, SVR [spleen volume reduction] of 35%, and symptom improvement with 50% reduction or more. Approximately 30% to 40% achieved that SVR of 35% [with ruxolitinib], and approximately 97% of the patients had spleen response [in COMFORT-II].
The median [length of] response [in] the COMFORT studies is approximately 3 years.3,4 We define primary failure as complete lack of response. Some [individuals] say 10% to 25% spleen reduction or lack of total symptom improvement. There is suboptimal response, and then there is a secondary failure where patients have a response and lose the response after that. In terms of the symptom response, there is no doubt that patients on ruxolitinib did better.4 Symptom response typically happens very quickly; within a couple of weeks, we see those patients getting better. Spleen response takes time. Symptom response is not dose dependent, but spleen response is dose dependent.
One important thing is if those patients are responding to treatment and [are] hospitalized and you are consulted on those patients, if you can continue or maintain the treatment, please do. Because if you stop, especially in the setting of pneumonia, infection, or sepsis, those patients get very sick because they get this phenomenon of withdrawal from ruxolitinib or a quick recurrence of their cytokine storm because the drug is off.
If you cannot [continue treatment] or those patients are not able to take oral medications, we bridge them sometimes with steroids. That is unique with ruxolitinib, and if we are stopping the ruxolitinib, particularly in patients who are responding, we taper; we do not stop suddenly.
What was the survival in the COMFORT trials?
[Individuals] argue about the survival. Those 2 studies allowed crossover, so they never looked at the survival.3,4 I think there is convincing evidence that those patients drive survival advantage. I always tell patients that I am not starting a JAK2 inhibitor because it improves survival. If somebody is completely asymptomatic [with] no spleen [and] early myelofibrosis, you cannot argue that ruxolitinib is going to alter the natural history. It does not reverse the fibrosis in the majority of patients or affect the molecular status of the disease. But if patients have intermediate- or higher-[risk disease] with spleen size and symptoms and they get treated with ruxolitinib, compared with historical hydroxyurea [Hydrea], their survival is improved. That is established at this point, and [individuals] accept that those medications do improve the survival for patients with myelofibrosis. Data from the European Registry for MPNs [also] showed that hydroxyurea was worse than ruxolitinib in overall survival.5
The spleen serves as a surrogate for survival, and if patients achieve spleen response at 3 and 6 months, that does correlate with survival.6 And what is the cutoff? It is suggested as 25% or more. Why is that? Some [individuals] think that the spleen response is a surrogate for the overall response, but the more I have seen this, [the more] I think there is a portion of patients whose mortality is related to long-term splenomegaly, where they get portal hypertension, cirrhosis, and ascites. I have seen several patients in the past couple of years who have developed cirrhosis and need abdominal paracentesis every week because of ascites and portal hypertension. There is a good correlation now between spleen response and survival in patients with myelofibrosis.
How does the dose of ruxolitinib affect patients’ response and adverse events (AEs)?
When you look at spleen reduction, you see a dose-dependent [change].7 Most of the time, you need more than 10-mg dosing twice a day of ruxolitinib for spleen response, and it typically takes 3 to 4 months to get that response, [whereas] symptom response can be seen sometimes with as low as 5 mg twice daily.
Most of the AEs are hematologic: anemia, thrombocytopenia, and less frequently, neutropenia.3,4 Week 8 is the nadir. Most of the time we adjust the dosing. The counts partly stabilize because we back off the dosing. Other than myelosuppression, patients can get headache, recurrent urinary tract infection, or upper respiratory tract infection. But in general, the treatment is tolerated. Most patients are feeling better. There is rare herpes zoster reactivation. We do not do primary prophylaxis, but if patients have a reactivation, then we put them on after that.
Patients who continue ruxolitinib for a long time sometimes get atypical infections. This is a drug that affects T-cell function. It is even approved for graft-vs-host disease post transplant. So in patients who were on ruxolitinib for a long time, I have seen some atypical infections [such as]…cutaneous [malignant tumors] other than melanoma, basal cell carcinoma, and squamous cell carcinoma, which is a phenomenon related to MPN and any treatment we do, not just ruxolitinib.
How have patients with higher platelet counts done when receiving ruxolitinib?
The EXPAND study [NCT01317875] looked at ruxolitinib in patients with a platelet count of 50 to 100 × 109/L.8 The package insert allows 50 × 109/L and above, so this looked at starting at 5 mg and then escalating the dose; 10 mg twice daily was the maximum dosage you could go to. They have shown there are responses to this approach.9
In my practice, I struggle with patients who have borderline thrombocytopenia [obtaining] a meaningful dose of ruxolitinib. I think in patients with [a platelet count between] 50 to 100 × 109/L, it is very hard to get to that meaningful dose, and nowadays, I prefer to shift to pacritinib in that group of patients. Anemia does happen and gets worse with ruxolitinib.3
Patients, on average, will [experience a] drop in hemoglobin level by 2 g. But the responses in spleen and symptom improvement were the same whether the patient had anemia or not. So it does not decrease the response rate, but it is going to render patients transfusion dependent. If that is a short-lived thing, maybe it is OK. But if it is a long-term thing, I struggle with that a little, particularly if patients are not [undergoing] transplant. Nowadays, we do have other agents.
I [recommend] the approach of considering ESA [erythropoiesis-stimulating agent] early on for those patients to prevent them from becoming transfusion dependent. ESA is probably [appropriate] in a small subset of patients. [Start] thalidomide early if you are going to stick to ruxolitinib. But with other JAK2 inhibitors, for those patients we could also consider that. That is how I think of it.
Can you discuss the data for fedratinib from the JAKARTA trials?
Fedratinib is approved by the FDA.10 This drug went through 2 trials, JAKARTA [NCT01437787] and JAKARTA2 [NCT01523171].11-13 One of them was second line and the other was up front. Fedratinib does have spleen response.11,12 It has never been compared head-to-head with ruxolitinib but shows similar responses.
It’s probably a little bit gentler on the counts, although when you look at the trials, they were like apples and oranges. The thresholds for platelet counts and anemia were not the same, but nonetheless, it is a little gentler on the counts. Fedratinib has taken its place as maybe second-line [treatment] in patients with proliferative [disease], so if patients do not have cytopenias who are on ruxolitinib, then we could use fedratinib.
In the JAKARTA2 trial that looked at the second line, there [was an approximately] 20% to 30% response rate after ruxolitinib failure.13 The toxicity [showed] some cytopenias and gastrointestinal toxicity because of the FLT3 activity.13 The major thing that set back the approval of fedratinib was Wernicke encephalopathy.11 That originally led to holding back submission of the drug. When they looked at those cases in more detail, they were not as common. The real Wernicke encephalopathies were only a couple of cases and mostly in patients who had malnutrition or vomiting. But nonetheless, it is now a black box warning.10
You have to check thiamine levels at baseline. We give patients thiamine replacement. I have not seen any cases in my limited experience with fedratinib, but we’ve treated a handful of patients. I have not seen it, but it is something to keep in mind with the fedratinib.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Myeloproliferative neoplasms, version 3.2022. Accessed April 7, 2023. https://bit.ly/2Wcczfa
2. Jakafi. Prescribing information. Incyte; 2023. Accessed April 7, 2023. https://bit. ly/3UeXnLD
3. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/ NEJMoa1110557
4. Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. doi:10.1056/ NEJMoa1110556
5. Guglielmelli P, Ghirardi A, Carobbio A, et al. Impact of ruxolitinib on survival of patients with myelofibrosis in the real world: update of the ERNEST Study. Blood Adv. 2022;6(2):373-375. doi:10.1182/bloodadvances.2021006006
6. Palandri F, Palumbo GA, Bonifacio M, et al. Durability of spleen response affects the outcome of ruxolitinib-treated patients with myelofibrosis: results from a multicentre study on 284 patients. Leuk Res. 2018;74:86-88. doi:10.1016/j.leukres.2018.10.001
7. Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13-21. doi:10.2147/OTT.S53348
8. Vannucchi AM, Te Boekhorst PAW, Harrison CN, et al. EXPAND, a dose-finding study of ruxolitinib in patients with myelofibrosis and low platelet counts: 48-week follow-up analysis. Haematologica. 2019;104(5):947-954. doi:10.3324/haematol.2018.204602
9. Guglielmelli P, Kiladjian JJ, Vannucchi AM, et al. The final analysis of EXPAND: a phase 1b, open-label, dose-finding study of ruxolitinib (RUX) in patients (pts) with myelofibrosis (MF) and low platelet (PLT) count (50 × 109/L to < 100 × 109/L) at baseline. Blood. 2020;136(suppl 1):4-5. doi:10.1182/blood-2020-137742
10. Inrebic. Prescribing information. Bristol Myers Squibb; 2022. Accessed April 7, 2023. https://bit.ly/3ZNN4PO
11. Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643-651. doi:10.1001/jamaoncol.2015.1590
12. Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
13. Harrison CN, Schaap N, Vannucchi AM, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317-e324. doi:10.1016/S2352-3026(17)30088-1

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More