CASE SUMMARY
Eight years ago, a 63-year-old man from a rural community received a diagnosis of multiple myeloma (IgG-κ). He recently presented with penta-refractory disease progression after 4 prior lines of therapy that included autologous stem cell transplant, 2 proteasome inhibitors, 2 immunomodulatory drugs, and 1 anti-CD38 antibody. He had hyper-tension controlled with lisinopril and an ECOG performance status of 1.
The patient asked about chimeric antigen receptor (CAR) T-cell therapy but after counseling opted to pursue an alternative therapeutic because of waiting lists at accessible institutions. He was eligible to receive teclistamab-cqyv (Tecvayli), a bispecific T-cell engager.
DISCUSSION QUESTIONS
- What is your reaction to the safety data for teclistamab from the MajesTEC-1 trial (NCT04557098)?
- Now that this therapeutic has been approved by the FDA, how likely will you be to administer it or refer to another institution? Would you administer cycle 1 in the community oncology setting?
- Would you administer later cycles in patients who receive cycle 1 at an academic center but prefer to receive their care close to home?
MODERATOR SIDEBAR
Targeted Oncology: What adverse events (AEs) were associated with teclistamab in patients with relapsed/ refractory multiple myeloma in the MajesTEC-1 study?
KUMAR: [In addition to] cytokine release syndrome [CRS], what limits easy adoption to practice this across the board is the presence of some unique toxicity related to the immune effector cell approach. Almost all patients had some AEs.1 Nearly all patients [95%] had at least 1 grade 3 or grade 4 event, but the majority of the patients did not end up discontinuing therapy because of AEs. Only 2 patients discontinued. There were some dose reductions and skipped doses related to AEs—more commonly skipped doses, in nearly two-thirds of the patients.
[Over] 70% of the patients had CRS, but most of them were grade 1 or 2. Grade 3 CRS was seen in 1 patient. The second set of AEs that are fairly common across the board are hematologic. You see a significant amount of neutropenia, but the majority of the patients with cytopenias do start resolving as they continue on therapy. Then you have other gastrointestinal AEs and injection site reactions.
We need to be aware of…the risk of infections. Nearly three-quarters of the patients had some infection and almost half of the patients had grade 3 and grade 4 infections, and this is quite high in the context of other agents that have been studied in multiple myeloma.
In addition to the CRS, the other [AE of note] is ICANS [immune effector cell–associated neurotoxicity syndrome]. It is about 6% [in this trial], much lower than what we see with CAR [chimeric antigen receptor] T cells, but nevertheless it is something that we need to keep an eye out for as we take these patients through initial therapy. Neurologic toxicities include headache, motor dysfunction, sensory neuropathy, and encephalopathy. Thankfully all these things are reversible.3
We haven’t seen neurologic toxicity like the parkinsonism we have seen with ciltacabtagene autoleucel [cilta-cel; Carvykti].4 None of that has been reported in the context of any of the bispecifics so far. Typically, we would halt therapy until [neurologic] AEs start subsiding.
What approaches can providers take to manage these toxicities?
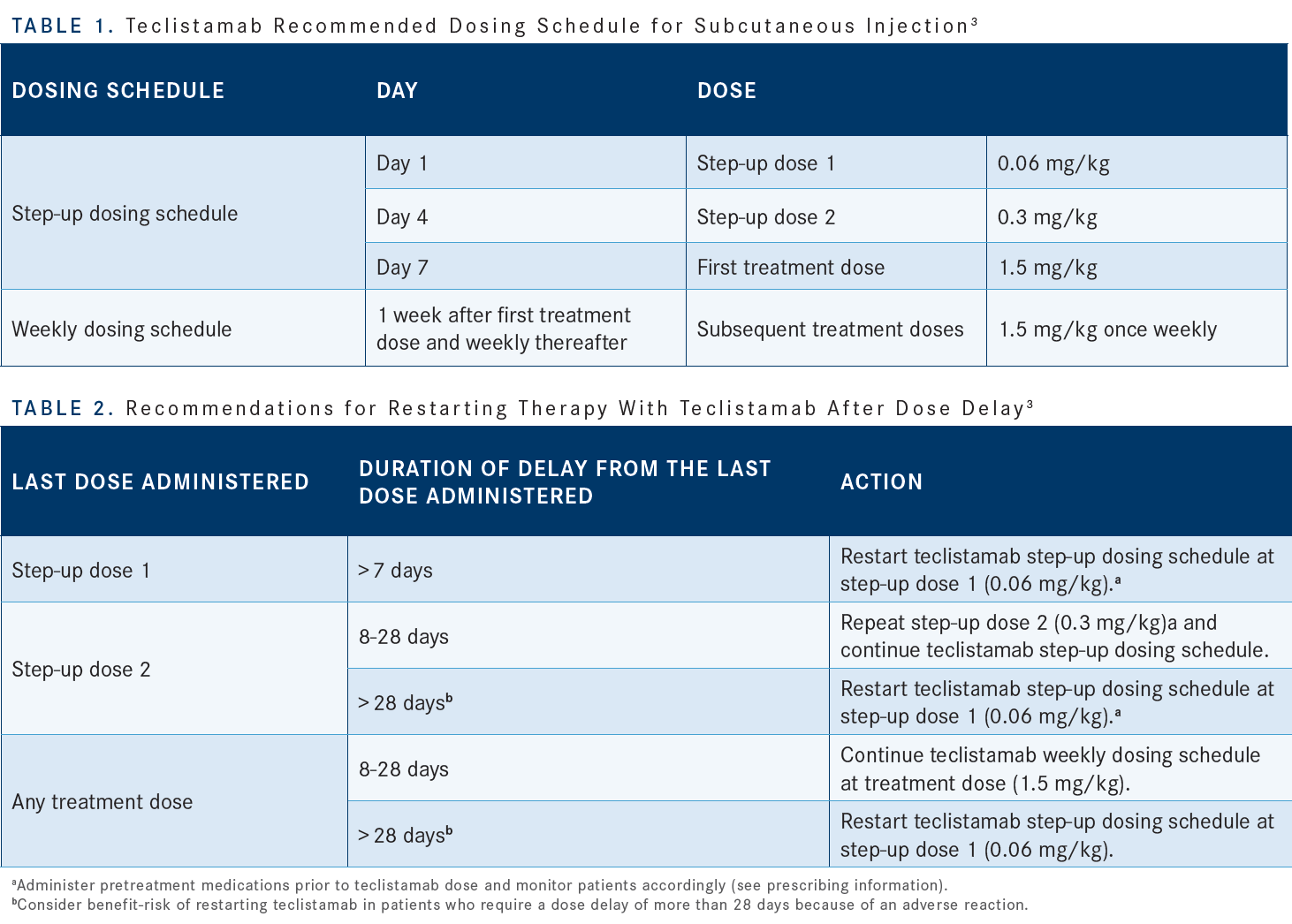
You have to watch these patients closely [for] CRS management. The injection reactions have to be kept in mind as well.3 One of the tactics that have been taken for most of the bispecifics to avoid or reduce the frequency of CRS is to use a step-up dosing schedule [Table 13]. In this case, we have a day 1 dose which is small, then a slightly higher dose day 4, and day 7 they get the full dose. Teclistamab is [then] given subcutaneously once weekly.
We use the typical infusion or injection prophylaxis that includes a steroid, antihistamines, and antipyretics. The REMS [Risk Evaluation and Mitigation Strategy] program suggests that the patients should be hospitalized.5 As long as you can monitor the patients closely, you don’t have to put them in the hospital. In our own practice, we are trying to do this as an outpatient [therapy] with the use of remote patient monitoring tools. These patients will have pulse and temperature sensors that are placed on them when they leave the outpatient area so then we can monitor them closely, and they can come back quickly if they have fevers. These patients will continue to be seen in the outpatient setting on a daily basis until…after the first couple of weeks.
There are no dose reductions per se, but you can delay the dose if they have toxicities. If there’s a significant delay at any time point, then you have to restart with the step-up dosing schedule [Table 23].
KUMAR: Do you think you will be starting it from cycle 1 or would you rather have them get it elsewhere at a larger institution and then come back to you for the remaining doses?
JI: I think this is an exciting drug. For community oncologists, it will be a learning curve. I don’t think we can always refer to another institution just to do cycle 1 and [then] we take over. We learned how to give patients daratumumab [Darzalex]. Initially, there were a lot of infusion reactions. Now everybody feels very comfortable and we give it to patients all the time.
Most patients [receiving teclistamab] get cytokine release syndrome [CRS] but the [rate of] grade 3 and 4 CRS is pretty low.1 Most of us can probably learn how to give the patients the medication. My concern is the infection rate, [which] is high. In addition to giving them IVIG [intravenous immunoglobulin], do you think giving them prophylactic antibiotics will help?
KUMAR: It has not been studied systematically in any of the studies, but it’s a reasonable suggestion to consider prophylactic antibiotics. The caveat is it’s not necessarily just bacterial infections.
You’re seeing other types of infections, so the key thing would be to have a high index of suspicion if patients come in with symptoms that aren’t well explained and make sure infections are ruled out. Going back to your initial comment about CRS, I completely agree. I think [we] are getting familiar with this toxicity, and the majority of these patients are grade 1 or 2. Only about a third of the patients have received tocilizumab [Actemra] in the clinical trials with these bispecific antibodies.1 There were some data presented at ASH [2022 American Society of Hematology Annual Meeting] looking at the concept of prophylactic tocilizumab administration.
At least in the context of the cevostamab [FcRH5 x CD3] trial [NCT03275103], they showed that the proportion of patients getting CRS, if you prophylactically gave tocilizumab before the first dose, was almost halved.2 There are other attempts looking at drugs to see if you can reduce the risk of CRS. That is one thing that’s going to help increase community adoption.
Many of these clinical trials enroll patients with high tumor burden. In our own practice, we are probably never going to wait for patients to get to that point before we start them on therapy. Hopefully, we are going to see less CRS in our own practice than in the clinical trial. As you said, it’s a learning curve. I am sure many of you use blinatumomab [Blincyto] in practice and it took a while for everybody to get used to that.
NAQVI: [There were] 19 deaths [related to adverse events (AEs)]; a mortality rate of more than 10% would make me very nervous to use it even [only] after the first cycle at this point. I would like to see a bigger trial and more information in this patient population.
KUMAR: You bring up a good point, but also you have to take it in the context that 12 of the 19 were COVID-19– related deaths.1
NAQVI: That’s correct, but we have treated many cancers during the COVID-19 pandemic in the last 3 years. We haven’t seen [a similar death rate in other settings] so the underlying mechanism is profound immunosuppression of patients with multiple myeloma [plus] what was induced by this drug. We have to keep that in mind right now until we see more data on this.
KUMAR: I couldn’t agree more. We need to have a high index of suspicion or threshold for investigating these patients for infections. The high rate of infections is multifactorial, but a bulk of the responsibility goes toward the drug and its mechanism of action.
But other things will play [a role] as well because before many of these immunotherapies came along, we didn’t have patients with penta-refractory disease surviving very long. If you look at the natural history of the disease, the median overall survival for patients who were enrolled in this trial would have been in the range of 8 to 9 months, and here these patients are living a lot longer with a very susceptible immune system.
If you ask me [whether] the infection alone would stop me from using the drug, my answer would be no. I would still use it very carefully, looking out for infections, but we have to discuss with patients in great depth before we put them on the drug.
GUPTA: When do the patients get infection most? Is it the first week when they’re getting the drug, or do they get infections later?
KUMAR: No, it seems to be throughout the course of therapy. Initially there is certainly a higher rate and that is probably [caused by] the uncontrolled disease plus the immunosuppression. But even after the disease comes under control, we see infections in these patients.
GUPTA: If there was more risk of infection during the first week or second week [and] you needed a special unit where the patient should get it, like for a bone marrow transplant, that would be different. But otherwise…the point of having bispecific antibodies is that they can be given in peripheral institutions rather than tertiary centers.
KUMAR: One of the important things we need to understand better is how long we need to give this treatment. There are trials looking at trying to give it less often as we do the longer phases of the therapy and also stopping at some point in time. Both of those will also help with the AEs.
VEGUNTA: What infection prophylaxis do you give for these patients?
KUMAR: We certainly need to put them on for PJP [Pneumocystis jirovecii pneumonia] prophylaxis, and all these patients should be on acyclovir as well. If they’re going through neutropenic phases, then a fluoroquinolone prophylaxis would be appropriate for that point in time. And if patients come in with unexplained fevers or cytopenias that are persistent, checking them for CMV [cytomegalovirus] or EBV [Epstein-Barr virus] would be appropriate as well. Patients with preexisting chronic hepatitis infections should be put on therapeutic doses of antivirals.
ELKADI: Do you replace the IgG for patients who have hypogammaglobulinemia? Do you give them IVIG? If so, do you check it regularly and do you replace it monthly or so?
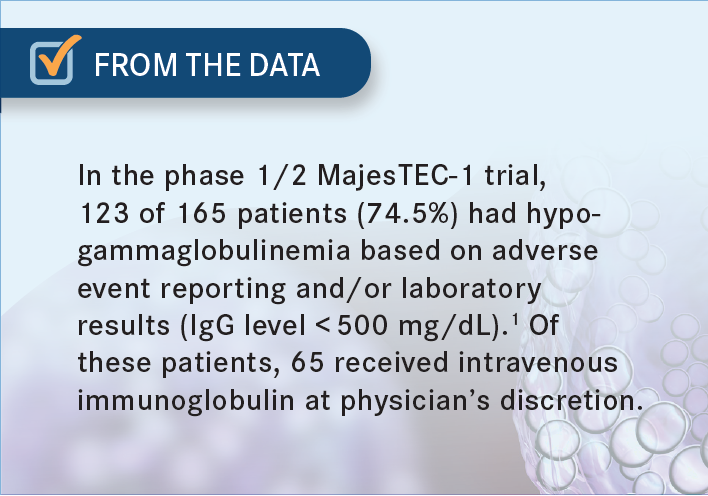
KUMAR: Absolutely, we do check. With both bispecifics and CAR T-cell therapy, we should be monitoring the IgG levels and replacing it. Our approach has been if they fall under 400 mg/dL then we give them IVIG infusion [From the Data1]. Sometimes it can be challenging when you have patients with IgG myeloma. We don’t know how much of that is normal IgG vs myeloma. In a rough calculation, you can subtract the M spike from the total IgG and it gives you the normal IgG.
THAW: In terms of management, is cycle number 1 going to be different from other cycles or is it expected to be a similar AE profile?
KUMAR: The CRS and the ICANS [immune effector cell– associated neurotoxicity syndrome] are typically seen only with the initial couple of weeks, maybe [only] the first cycle. Beyond that, unless they have a prolonged interruption of therapy, we don’t typically see those AEs again.
REFERENCES
1. Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495-505. doi:10.1056/ NEJMoa2203478
2. Trudel S, Bahlis NJ, Spencer A, et al. Pretreatment with tocilizumab prior to the CD3 bispecific cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) showed a marked reduction in cytokine release syndrome incidence and severity. Blood. 2022;140(suppl 1):1363-1365. doi:10.1182/ blood-2022-159381
3. Tecvayli. Prescribing information. Janssen Pharmaceutical Companies; 2022. Accessed April 3, 2023. https://bit.ly/3ZwQJBr
4. Carvykti. Prescribing information. Janssen Pharmaceutical Companies; 2022. Accessed April 3, 2023. https://bit.ly/3Uca1ek
5. Tecvayli risk evaluation and mitigation strategy (REMS). Janssen Biotech Inc. Accessed April 3, 2023. https://bit.ly/3m3pVLm