Park Discusses Targeted Therapy Trials in Intermediate- and High-Risk ccRCC
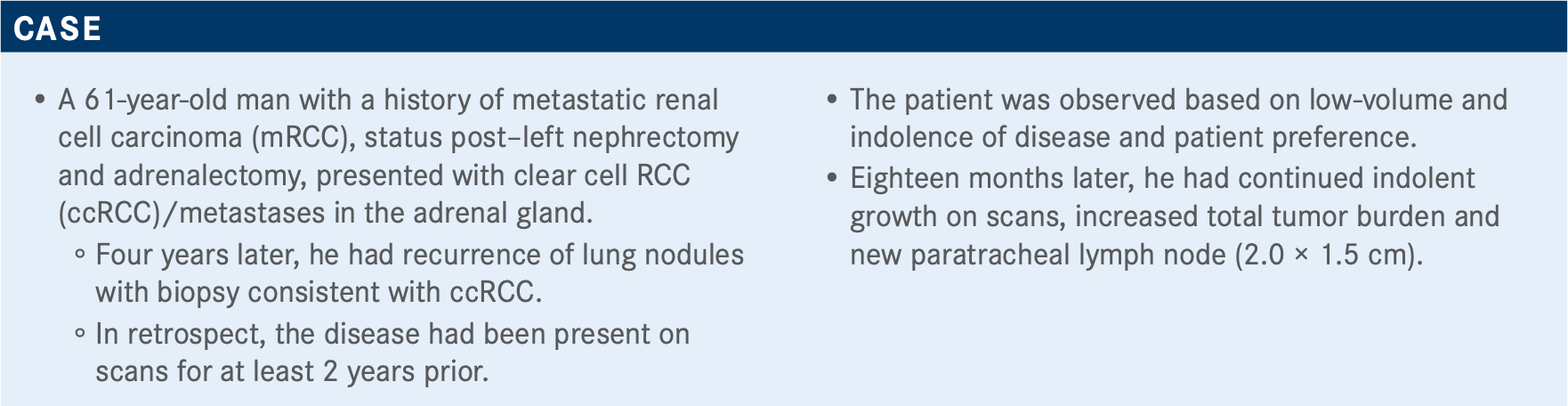
In a 7 to 1 vote, oncologists favored the use of axitinib in combination with pembrolizumab or avelumab over cabozantinib monotherapy for the treatment of intermediate and high-risk clear cell renal cell carcinoma. Chandler H. Park, MD, reviewed that clinical trial research that supports the axitinib combinations, as well as other targeted therapy options.
Chandler H. Park, MD

In a 7 to 1 vote, oncologists favored the use of axitinib (Inlyta) in combination with pembrolizumab (Keytruda) or avelumab (Bavencio) over cabozantinib (Cabometyx) monotherapy for the treatment of intermediate and high-risk clear cell renal cell carcinoma (ccRCC). Chandler H. Park, MD, the codirector of Genitourinary Clinical Trials at Norton Cancer Institute, reviewed that clinical trial research that supports the axitinib combinations, as well as other targeted therapy options.
Targeted OncologyTM: What are some things you would consider for patients who have favorable risk to initiate systemic therapy?
Based upon the International Metastatic RCC Database Consortium [IMDC] risk profile, this patient has no risk factors.1
I think from my practice, for patients who have favorable risk, based upon the IMDC criteria...the patient [would be] started on a tyrosine kinase inhibitor [TKI]. In a Journal of Clinical Oncology paper, patients were started on sunitinib [Sutent]. Then the question is, how many of those patients get a complete response [CR]? It's very low, maybe 1%. And so you can have a patient with favorable response with mRCC and still have some burden of disease. There are some patients I wouldn’t treat. Otherwise, if I was going to do treatment, I would go with the higher pembrolizumab [Keytruda] with axitinib [Inlyta] because there is a higher CR rate. That’s just my personal preference.
Would you choose the same or differently from the participants?
I agree with everybody. The data for the axitinib and pembrolizumab included all comers: patients with high risk, intermediate risk, and also favorable risk.2 The nivolumab [Opdivo]/ipilimumab [Yervoy] [was investigated only in patients with] high risk and intermediate risk.3 Sunitinib is also a possibility here because it’s considered in the IMDC criteria, by definition, that patients [could be] treated on that, as well.4
Would you consider continuing with a TKI such as cabozantinib [Cabometyx] in the first-line setting? Or are there other options you would prefer for patients who have favorable risk?
On the cabozantinib trial, by definition in that study, they only [investigated] the high-risk and the intermediate-risk population.5 I think you can treat [with that], but I rely on the data.
Now, in terms of the sunitinib versus axitinib [plus] pembrolizumab, this is a heterogeneous population of patients who have favorable risk.2 The patients who have favorable risk can have high burden of disease. They might be symptomatic versus asymptomatic...patients with favorable risk [could] have 1 or 2 lesions in the lung. Believe it or not, I wouldn’t treat that person—I would watch because they have a low volume of disease. Now, on the other hand, if they have a high burden of disease, which is still possible with the IMDC criteria, I’m [going to try] to control the cancer. The overall survival [OS] hazard ratio [for patients who have favorable risk receiving pembrolizumab and axitinib] is very controversial. It does cross 1.00; it’s not statistically significant.2
If a patient doesn’t have high-volume disease and you were considering doing monotherapy with sunitinib, I think that would be a fair choice. My only concern with sunitinib, even though the data are there, is that it’s rare to see somebody get a CR on treatment with sunitinib.
What are the primary factors that drive your selection of frontline therapy for patients with primary favorable risk mRCC or ccRCC?
Volume of disease—if we have 1 or 2 spots in 1 lobe of the lung, we have to consider primary treatment with stereotactic body radiation therapy versus surgery; volume of disease, whether the patient is young, the data are not strong, with the hazard ratio crossing 1.00, but you still have to think about patients getting the CR with the dual treatments.
I think the greater question is, do you treat or not? The IMDC favorable risk OS, progression-free survival [PFS], and overall response rate [ORR] in KEYNOTE-426 [NCT02853331] showed an OS hazard ratio of 1.06 [95% CI, 0.60-1.86]; it is not statistically significant with the study arm [of pembrolizumab and axitinib versus] with sunitinib.6 The PFS hazard ratio was 0.79 [95% CI, 0.57-1.09]. The ORR with pembrolizumab and axitinib [was 69.6% and] an 11% CR rate versus 6% CR rate with the sunitinib, and so that becomes a tough choice.
How do the approved first-line TKI trials compare?
The sunitinib phase 3 trial randomized 750 patients compared to interferon [and included patients with] all risk factors.4 The median PFS was 11 months with sunitinib versus 5 months [HR, 0.42; 95% CI, 0.32-0.54; P <.001]; median OS was 26.4 months versus 21.8 months [HR, 0.82; 95% CI, 0.67-1.00; P =.051].
Then we had the New England Journal of Medicine paper [which] was a noninferiority trial comparing pazopanib [Votrient] versus sunitinib [in all risk].7 [The median PFS was] 8.4 months [with pazopanib] versus 9.5 months [with sunitinib (HR, 1.05; 95% CI, 0.90-1.22)]; median OS was 28.4 versus 29.3 [HR, 0.91; 95% CI, 0.76-1.08; P =.28]; ORR was 31% versus 25%. I think once the paper came out, more providers started using pazopanib because of the treatment adverse effect profile.
What was the efficacy of nivolumab and ipilimumab compared with sunitinib?
The study designed for CheckMate 214 was for patients with treatment-naïve RCC clear-cell component, measurable disease, stratified by IMDC criteria: favorable, intermediate, or poor risk.
The key point here is that we all agree, patients not should not be treated with nivolumab and ipilimumab for favorable risk because the data [do not support it].3
How would you treat this patient in the second line?

I think...I would go with an immune checkpoint inhibitor in the second line. Nivolumab is approved second line, but I would lean more toward nivolumab with ipilimumab if you can get insurance approval. I would go with immunotherapy second line because this patient] is immune therapy naïve.
Has nivolumab been studied in other capacities for this population?
[Nivolumab was evaluated in] CheckMate 9ER [NCT03141177]— the key inclusion criteria were previously untreated advanced or mRCC clear cell component.8 It included all risk factors. Patients were randomized to nivolumab 240 mg intravenously every 2 weeks plus cabozantinib, typically started at 60 mg, but in this study started at 40 mg [by mouth daily]. The comparison arm once again was sunitinib, and the primary end point was PFS. The secondary end points were OS and ORR.
What were the efficacy and safety of the CheckMate 9ER trial?
The PFS around the 3-month mark showed clear separation [of Kaplan-Meier curves]. There was a hazard ratio of 0.51 [95% CI, 0.41- 0.64; P <.0001]. The median PFS was 16.6 months and 8.3 months [with nivolumab/cabozantinib versus sunitinib]. The subgroup analysis for favorable, intermediate, and poor risk—everything [favored the study drug]. It has strong data for frontline treatment for PFS.
OS hazard ratio was 0.60 [98.89% CI, 0.40-0.89; P =.001]. Since this is an early study, [the median for either arm was not reached] yet. But there was a separation of the curve with clear meaningful differences there.
CheckMate 9ER ORR versus best overall response [showed a] 56% response rate with the nivolumab/cabozantinib versus 27% with sunitinib. We can’t compare studies, but from the outside, if you look at the 3 studies—KEYNOTE-426 with pembrolizumab, [CheckMate 214 and CheckMate 9ER]—were around a 60% response rate. Then ipilimumab and nivolumab had about a 40% response rate....CR was about 8% with nivolumab/cabozantinib.
Now, this is an early [readout of the] study. With the KEYNOTE-426 study, the early readout, [CR] was around 5% to 6%. But then at the updated presentation, it went up to 9% to 10%. Partial response was 47.7% and stable disease was 32.2% with nivolumab/cabozantinib. This regimen definitely has activity [in this population].
The median duration of treatment was 14.3 versus 9.2 months. [The proportion of] patients with at least 1 dose reduction was 56.3%; the patients went from 40 mg to 20 mg [of cabozantinib]. With 20 mg, you still have activity, but you can’t go below 20 mg. Treatment discontinuation was 44.4% [with the study drugs]. Treatment discontinuation due to disease progression was 27.8% versus 48.1%. [The rate of] any grade treatment-related adverse effects leading to discontinuation of nivolumab only was 5.6%; cabozantinib, 6.6%; and both nivolumab/cabozantinib, 3.1%.
The safety profile showed diarrhea at 57% in the nivolumab plus cabozantinib arm and 43% on sunitinib. The half-life of cabozantinib is 55 hours, which is a bit different from axitinib, [which has] 3 to 4 hours of half-life. So we were talking about how to separate the diarrhea from immunotherapy and TKIs. It’ll be a bit tougher because cabozantinib does cause diarrhea. The hand/foot syndrome was similar with both arms; hypertension was also similar.
What are your reactions to the introduction of new immunotherapy and VEGF TKI regimens?
I think the key for all of us to think about is, there are some good studies that show that CTLA-4 tends to have the most activity on the front line. There are equally strong data that show that the VEGF has the most activity in the front line. So it boils down to which one you pick in the front line: Do you check the immunotherapy with the VEGF up front versus dual checkpoint inhibitor?
The other trial that’s going to be coming out, which is the CLEAR trial [NCT02811861], will come out at one of the [upcoming] American Society of Clinical Oncology meetings, and that’s going to be a 3-arm trial of lenvatinib [Lenvima] with everolimus [Afinitor] up front, which is an interesting arm. Then you have the lenvatinib and pembrolizumab arm, then the sunitinib arm. Most of the thought leaders believe that this is going to be a positive study.
Based upon the response rate, more [clinicians] are leaning more toward TKI versus immunotherapy. Now on the other hand, nivolumab/ipilimumab has the longest data in terms of OS, so this is going to be difficult in terms of the initial treatment, because you committed going down [a certain] road.
Let’s say you do the nivolumab/ipilimumab; what I foresee happening is the PDIGREE trial [NCT03793166] readout. Second line would be cabozantinib, and then cabozantinib versus cabozantinib and nivolumab because that will be the arm for the PDIGREE trial.
If the patient progresses...even though there are some data with lenvatinib and everolimus, cabozantinib seems to be really strong as a second option after the first line.
REFERENCES:
1.International mRCC Database Consortium. Accessed January 14, 2021. https://bit.ly/35IXYgx
2. Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
3.Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi:10.1056/NEJMoa1712126
4. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for suni- tinib compared with interferon alfa in patients with metastatic renal cell carcinoma.J Clin Oncol. 2009;27(22):3584-3590. doi:10.1200/JCO.2008.20.1293
5. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or interme- diate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591-597. doi:10.1200/JCO.2016.70.7398
6. Plimack E, Rini BI, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first- line therapy for advanced renal cell carcinoma (RCC): Updated analysis of KEYNOTE- 426. J Clin Oncol. 2020;38(suppl 15):5001. doi:10.1200/JCO.2020.38.15_suppl.5001
7. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal- cell carcinoma. N Engl J Med. 2013;369(8):722-731. doi:10.1056/NEJMoa1303989
8. Choueiri TK, Powles T, Burotto M, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase 3 CheckMate 9ER trial. Ann Oncol. 2020;31(suppl 4):S1142-S1215. doi:10.1016/annonc/annonc325
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen







