Combining Bevacizumab “Additive” But Not “Synergistic” With Olaparib, According to Moore
During a Targeted Oncology Case-Based Peer Perspective event, Kathleen Moore, MD, discussed the combination of bevacizumab and olaparib as treatment of ovarian cancer.
Kathleen Moore, MD

During a Targeted Oncology Case-Based Peer Perspective event, Kathleen Moore, MD, associate professor of Obstetrics and Gynecology, Jim and Christy Everest Endowed Chair in Cancer Research, The University of Oklahoma College of Medicine and associate director of Clinical Research, medical director of the Clinical Trials Office, Stephenson Cancer Center discussed the combination of bevacizumab (Avastin) and olaparib (Lynparza) as treatment of ovarian cancer.
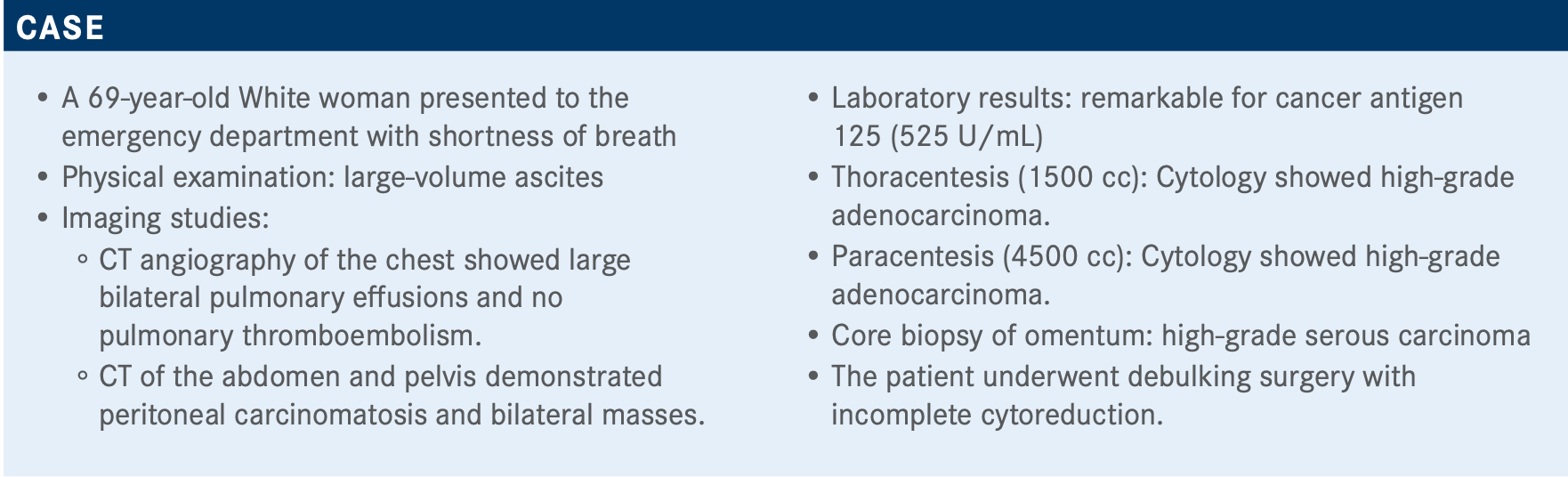
Targeted OncologyTM: What type of testing should be ordered for a patient with these symptoms and laboratory findings?
MOORE: You want to get testing launched because it is going to affect her decision for maintenance later. You want results back sooner rather than later. For germline testing, once you get her started on chemotherapy and feeling better, then you can start having those discussions about referral to genetic counseling for the whole family. In this case, the patient was sent off for BRCA testing.

What are your thoughts on the results of this poll?

Of those polled, 52% are sending it on all patients and 38% are testing it only if they have a negative germline BRCA. I think both are right. If you start with the tissue test, and especially if you send to Foundation Medicine, you’re going to get your somatic BRCA and you’re going to get your HRD together. It’s equally right to send your germline testing first, and if that’s positive, you don’t need to [test for HRD] because they already qualify as HRD and you know what you’re going to do. It might save you a little bit of time and money.
How do BRCA and HRD test results change treatment?
I’m a big proponent of primary cytoreduction. Sometimes someone can have a lot of disease, but we can still get it out. For this patient, she was stage IV with pleural effusion, so she needed neoadjuvant therapy. I use bevacizumab. I know the data [are] not as strong there, but patients respond quicker and feel better.
For neoadjuvant therapy, do you complete 6 cycles, or do you use 3 cycles, surgery, and then 3 more cycles?
There are data that appear to show [that] the earlier surgery is done, the better patients do. I try to go for surgery after 3 or 4 cycles. I like to get the primary [cancerous tissue] out and the omentum—just to make sure nothing is there—and then I’ll do 3 more cycles. If there is still disease after surgery, I’ll do 2 to 3 more cycles. I might take them to 9 cycles. There [are] no data for that, but that’s what I do. I try to get as best a response as I can before I put them on a PARP inhibitor.
What do the National Comprehensive Cancer Network guidelines suggest at this point for such a patient?

If bevacizumab was used as part of frontline therapy and the patient is BRCA wild type [with a] complete or partial response, bevacizumab can be used alone or with olaparib. If they have a BRCA mutation, options include monotherapy olaparib and monotherapy niraparib [Zejula], or bevacizumab can be continued with olaparib.1
Is there a reason to choose single-agent bevacizumab over bevacizumab/olaparib as maintenance therapy?
[A majority of my peers would say] adverse events [AEs] and cost. I also think [looking at] someone who is struggling with oral AEs, such as a lot of nausea and vomiting, [or] maybe someone who developed gastrointestinal toxicity, plays a role. And, unfortunately, we must remember financial toxicity to our patients is real.
If this patient was germline BRCA1/2 mutant, what would be offered as maintenance therapy after carboplatin/paclitaxel/bevacizumab primary therapy?
I think the situation that we’re going to face is [between] olaparib/bevacizumab and monotherapy olaparib. In the SOLO-1 clinical trial [NCT01844986], [which examined the efficacy of maintenance olaparib], the hazard ratio [HR] was 0.30, and in the PAOLA-1 study [NCT02477644; olaparib plus bevacizumab maintenance therapy], the HR was 0.33 in BRCA-associated cancers.2,3 Those were 2 unprecedented improvements in progression-free survival [PFS] when compared with placebo.
It’s hard to say what the best regimen is for BRCA-associated cancer. PAOLA-1 didn’t have an elaborate balloon arm.3 As part of the Society of Gynecologic Oncology, we did an exploratory analysis of PAOLA-1; we took the patients in PAOLA-1 that had BRCA-associated cancers and got bevacizumab and olaparib, and then we matched patients from SOLO-1 who got olaparib to [those PAOLA-1 patients].4 We tried to create a PAOLA-like population out of the patients who got olaparib and create that missing arm for PAOLA. Then we reran the PFS analysis. It’s completely exploratory. But the hazard ratio was 0.71. So it looked like there was an additive benefit of the bevacizumab in that particular patient population; not synergistic but additive.
Dropping bevacizumab and going [straight] to monotherapy with a PARP inhibitor would be taken into consideration if a patient were experiencing AEs. PAOLA shows us [AEs] are not insignificant, since 20% of patients had to stop treatment because of them.3
For a patient who was BRCA wild type and homologous recombination proficient [HRP], what maintenance therapy would be offered?
Some [oncologists] may want to keep the patient on bevacizumab, whereas others may want to switch to niraparib, which is indicated. I would want to use bevacizumab and olaparib.
[However,] that’s not indicated, so [insurance payers] could give push back.
Unfortunately, the VELIA clinical trial [NCT02470585]—which investigated if the addition of veliparib [ABT-888] to standard platinum-based chemotherapy and continued as maintenance therapy compared with chemotherapy alone prolonged PFS— is not being submitted for FDA approval.5 But it’s a big phase 3 study for the HRP group.
In PAOLA-1, bevacizumab looks the same as bevacizumab/ olaparib.4 For the PRIMA [trial; NCT02655016], niraparib looks better than nothing.6 For VELIA, veliparib looks better than nothing [by] a little bit.5 It's not statistically significant, but it is there.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2020. Accessed January 17, 2021. https://bit.ly/35ZX9PA
2. Moore K, Colombo N, Scambia G, Kim BG, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495- 2505. doi:10.1056/NEJMoa1810858
3. Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus beva- cizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416- 2428. doi:10.1056/NEJMoa1911361
4. Vergote LB, Moore KN, Hettle R, et al. Population adjusted indirect comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 studies of olaparib with or without beva- cizumab, bev alone and placebo in the maintenance treatment of women with newly diagnosed stage III/IV ovarian cancer with BRCA mutation. Presented at: Society of Gynecologic Oncology 2020 Annual Meeting on Women’s Cancer; March 28-31, 2020; virtual. Abstract 35.
5. Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403-2415. doi:10.1056/NEJMoa1909707
6. González-Martín A, Pothuri B, Vergote I, et al; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019:381(25):2391-2402. doi:10.1056/NEJMoa1910962
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More









