Bekaii-Saab Explores Chemotherapies and Targeted Treatment Options in Metastatic CRC
During a virtual Targeted Oncology Case-Based Peer Perspectives event, Dr. Tanios Bekaii-Saab discussed the chemotherapy and targeted treatment options available for the treatment of patients with metastatic colorectal cancer with participating oncologists.
Tanios Bekaii-Saab, MD

Targeted OncologyTM: Which factors affect the treatment of patients with colorectal cancer (CRC)? Which tests do you usually order in this setting?
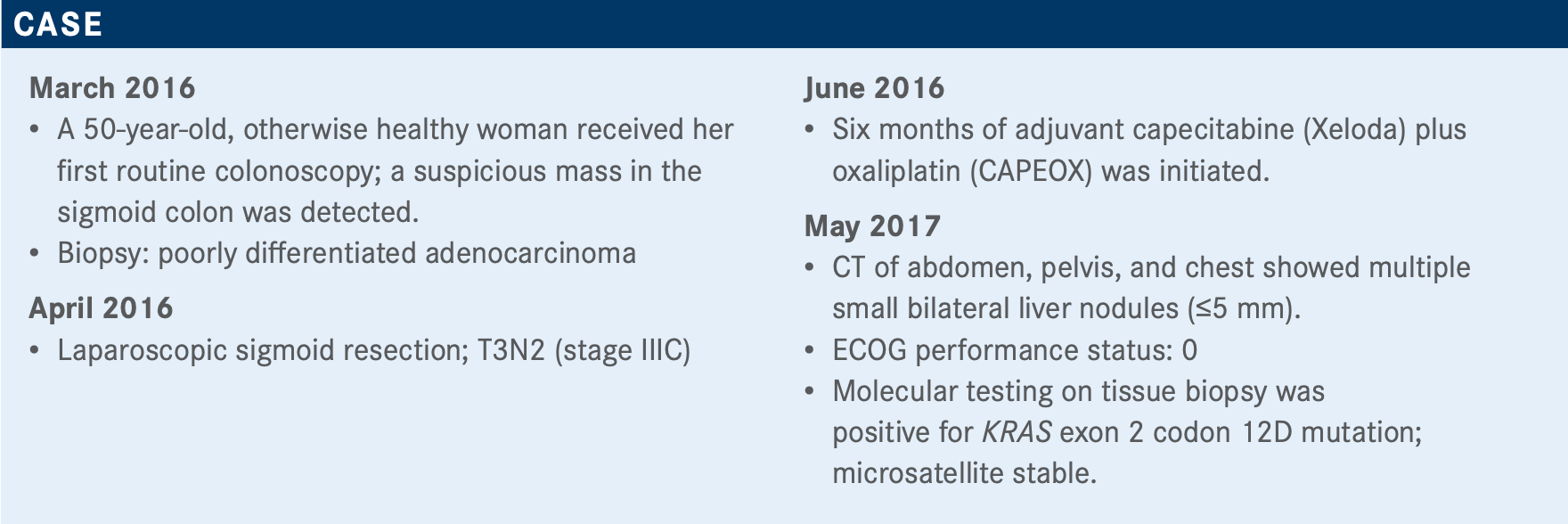
BEKAII-SAAB: The patient’s characteristics and age are important; whether they had prior adjuvant therapy; comorbidities; tumor burden. Is this a disease that you want to hit hard? Is this potentially resectable? It seems next-generation sequencing [is being done quite] liberally, but if you are limited by your choices, the first 4 [markers] you want on hand would be RAS, BRAF, microsatellite instability [MSI] high, and then HER2 amplifications. HER2 amplifications not only lead to HER2-targeted therapies at some point but also help eliminate the need for EGFR inhibitors, because HER2 amplifications predict for lack of efficacy of EGFR inhibitors. And, of course, patient preference [is also an important factor].
Which therapies do the National Comprehensive Cancer Network (NCCN) guidelines suggest?
The NCCN guidelines are a hodgepodge of [choices], and they really don’t limit you to 1 or 2 options. Pembrolizumab [Keytruda] is a first-line option for patients with MSI-high CRC given the results from KEYNOTE-177 [NCT02563002] that were presented at the American Society of Clinical Oncology [plenary session] and are now published as a paper in the New England Journal of Medicine.1
What is your impression of this patient’s case so far?
It’s reasonable to do FOLFOX, although the preference would be for FOLFIRI [folinic acid, fluorouracil, and irinotecan]. Twenty percent to 30% will have a response, regardless of prior exposure, to oxaliplatin, as long as it’s been a while.
The patient started developing peripheral neuropathy—not too bad, though. But at this point, the patient has had enough oxaliplatin to consider the response as plateaued.
An old paper suggests that it doesn’t matter how you sequence FOLFOX or FOLFIRI; you get about the same benefit, and the same is true today.2
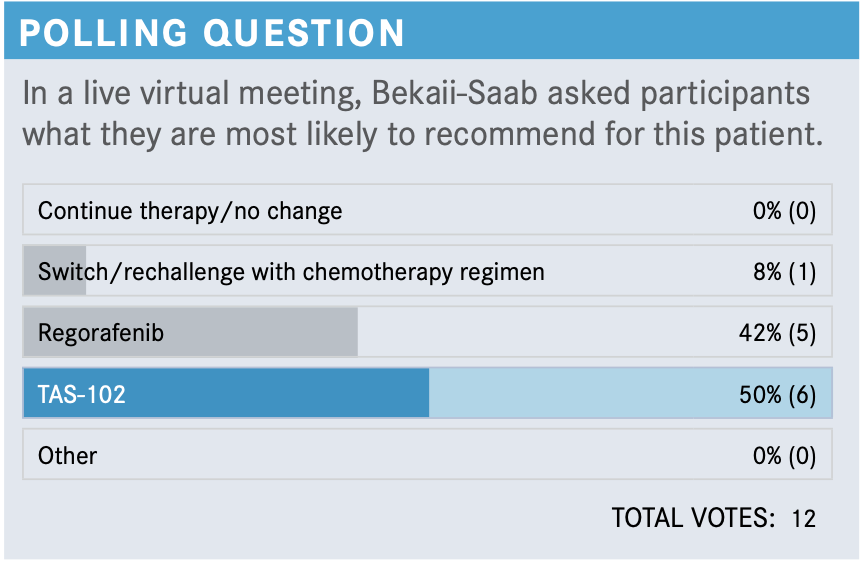
What are the data supporting the use of regorafenib (Stivarga) for patients with CRC?
The phase 3 CORRECT trial [NCT01103323] was the initial study with regorafenib, which showed a median overall survival [OS] of 6.4 months [with regorafenib] versus 5.0 months for placebo [HR, 0.77; 95% CI, 0.64-0.94; P =.0052].3 But the toxicities came early. In the first 2 weeks, you start seeing hand-foot syndrome, fatigue, and other toxicities like hypertension, which have limited the use. There have been a number of attempts to clinically [decrease these toxicities]; physicians have been using regorafenib every way they [could], starting with 80 mg or 120 mg, [to get to] 160 mg. There were not many data to help us understand a strategy to optimize the dose.
Some refer to [the ReDOS] study [NCT02368886] that we published in Lancet Oncology in 2019, and it was a large phase 2 randomized study looking at regorafenib.4 It was [increased] from 80 mg to 120 mg to 160 mg on a weekly basis, weeks 1, 2, and 3, and then with a week off versus 160 mg [daily for] 3 weeks on, 1 week off. The study’s primary end point was a composite end point of efficacy and tolerability. In other words, patients were accounted for if they essentially got to cycle 3 and then continued beyond cycle 3. It means that they tolerated the drug [well] enough and they responded well enough.
The primary end point of the study was positive, where the dose-escalation strategy was superior to the standard-dose strategy. The OS was surprising; this was a secondary end point, [and we] thought survival wasn’t going to look much better, but it did look better with the dose-escalation strategy versus the standard dose, at 10 months versus 6 months, [respectively; HR, 0.72; 95% CI, 0.47-1.10; P =.12].
This changed the NCCN guidelines, and [regorafenib was added] as an option. Not only has this become the preferred way to give regorafenib in the United States, but it has become adopted across most of the world.
Can you discuss the trials for TAS-102 (trifluridine/tipiracil; Lonsurf)?
TAS-102 came on the heels of the RECOURSE study [NCT01607957] randomizing patients to TAS-102 versus placebo, with a primary end point of OS.5 There were similar trends with RECOURSE OS and progression-free survival [PFS] to what we’ve seen with regorafenib. There has never been a formal head-to-head comparison, but the results look similar.
The toxicity profile was a bit different. You see mostly neutropenia. In fact, 4% of patients [on regorafenib] had febrile neutropenia. I had a couple of patients over the past few years who ended up with febrile neutropenia and were admitted to the hospital. We often think about oral medications as less toxic from that standpoint.
We published [another trial] in the Oncologist.6 This study essentially was an indirect comparison, a network meta-analysis. What we found, essentially, is what you would expect. If you look at regorafenib 160 mg versus TAS-102, it’s pretty much a wash. OS and PFS hazard ratios were very similar, not much difference between the 2 [OS HR, 1.00; PFS HR, 0.93]. We also took the regorafenib schedule, the 80-plus mg, and compared it indirectly with historical best supportive care to confirm that it will maintain that value. The hazard ratios are impressive with this [OS HR, 0.44; PFS HR, 0.37]. When we compared regorafenib 80-plus mg with 160 mg from all randomized studies, regorafenib 80-plus mg maintained its advantage versus regorafenib 160 [OS HR, 0.65; PFS HR, 0.89]. We compared 80-plus mg with TAS-102—the P values were not positive, so it was just a numerical improvement with the 80-plus mg. Our conclusion was that there is definitely a trend for improved survival when you look at regorafenib 80 mg, 120 mg, and 160 mg versus regorafenib 160 mg or TAS-102, to be confirmed in larger trials, if that ever happens.

REFERENCES
1. André T, Shiu KK, Kim TW, et al; KEYNOTE-177 Investigators. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207-2218. doi:10.1056/NEJMoa2017699
2. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229-237. doi:10.1200/JCO.2004.05.113
3. Grothey A, Van Cutsem E, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi:10.1016/S0140-6736(12)61900-X
4. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refrac- tory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi:10.1016/S1470-2045(19)30272-4
5. Mayer RJ, Van Cutsem E, Falcone A, et al; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. doi:10.1056/NEJMoa1414325
6. Sonbol MB, Benkhadra R, Wang Z, et al. A systematic review and network meta- analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer. Oncologist. 2019;24(9):1174-1179. doi:10.1634/theoncologist.2019-0189