Johnson Examines Osimertinib in the Frontline Setting for NSCLC
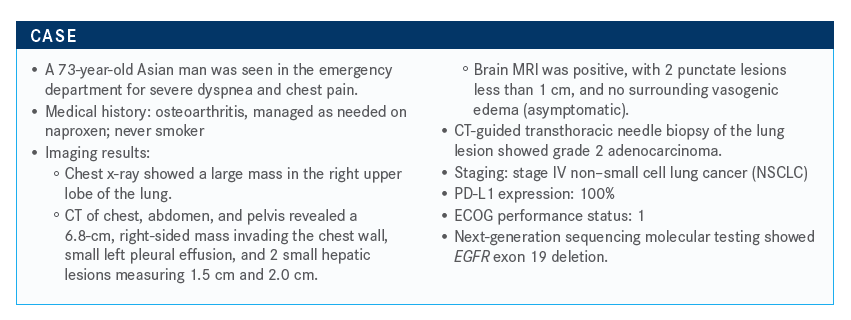
The case of a 73-year-old Asian patient diagnosed with non–small cell lung cancer and an EGFR exon 19 deletion was discussed during a virtual Case Based Peer Perspectives event, led by Melissa L. Johnson, MD.
Melissa L. Johnson, MD

The case of a 73-year-old Asian patient diagnosed with non–small cell lung cancer and an EGFR exon 19 deletion was discussed during a virtual Case Based Peer Perspectives event, led by Melissa L. Johnson, MD, associate director, Lung Cancer Research, Sarah Cannon, Tennessee Oncology, Nashville, TN.
Targeted Oncology™: What led to the approval of osimertinib non–small cell lung cancer?
JOHNSON: The FLAURA study [NCT02296125] led to the approval of osimertinib [Tagrisso].1 In this trial, 500 patients with stable brain metastases and EGFR exon 19 or 21—the most common EGFR activating mutations—were randomized 1:1. Patients who were randomized to the control arm could receive erlotinib [Tarceva] or gefi tinib [Iressa]. It was the investigator’s choice. The primary end point, as is the case in most of these trials, was PFS [progression-free survival], but response was also evaluated.
The PFS was 18.9 months for the treatment arm versus 10.0 months with erlotinib or gefitinib [HR, 0.46; 95% CI, 0.37-0.57; P < .001]. We see an improvement in the PFS as well as in the amount of time that patients are living without progression.
The median OS [overall survival] was 38.6 months in the osimertinib arm versus 31.8 months in the standard-of-care arm [HR, 0.63; 95% CI, 0.45-0.88; P = .0068]. For the patients who were randomized to receive TKIs [tyrosine kinase inhibitors], you had better believe they received osimertinib when they progressed. The crossover happened in a large percentage, which muddies the ability to show an OS result because they received the experimental drug, just later on down the road. But the hazard ratio of 0.63 and a significant P value show that it was important to receive osimertinib first.
What did the subgroup analysis show?
For the most part, all patients had an even higher, or more significant, hazard ratio in favor of…osimertinib. Regardless of whether [patients] had brain metastases at diagnosis, all had better survival when treated with osimertinib in the frontline setting.
Response rates were equivalent for the 2 regimens. But the point is they were close to 80%. That’s the reason I would say that [a patient with] EGFR-positive [disease] could have that Lazarus effect with this sort of chemotherapeutic. [The phenomenon is sometimes observed with the use of novel targeted therapies in patients with NSCLC who have oncogenic mutations along with poor performance status.]
Certainly, that’s not the case with methotrexate, or any other treatment, but this is a decent drug.
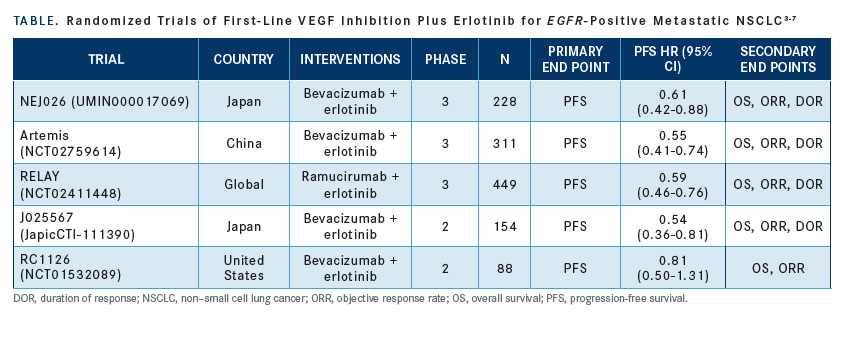
Can you add bevacizumab (Avastin) to an EGFR TKI and create synergy that will benefit patients over and above giving the TKI alone?
That’s a thought-generating question. At ASCO [American Society of Clinical Oncology] this year, we learned about a number of trials that explored this topic [TABLE on page 26].2 Results from these trials suggest that the addition of bevacizumab tends to help with PFS [but] maybe not with OS.3-7
What did the results of RELAY suggest?
The RELAY trial [NCT02411448] showed improvement in OS and PFS for patients who received ramucirumab [Cyramza] plus erlotinib.8 Median duration of response was 18.0 months for the treatment arm versus 11.1 months for the control [P < .005].
This was a 500-patient trial, and all patients had EGFR mutations. They received ramucirumab and erlotinib versus erlotinib alone, and the primary end point was PFS. After a median followup of 20.7 months, patients in the ramucirumab had a PFS of 19.4 months versus 12.4 months for the erlotinib arm [HR, 0.591; 95% CI, 0.461-0.760; P < .0001].8
When adding ramucirumab or bevacizumab to another regimen, there is a slightly higher risk of bleeding. Overall, there’s not much toxicity in terms of grade 3 or 4 adverse effects. Patients in the ramucirumab arm did exhibit more cases of grade 3 hypertension than patients in the erlotinib arm.
Given the RELAY data, where does ramucirumab fit into your treatment in the frontline setting?
The challenge with this question is that osimertinib is our practice standard, so it’s not osimertinib plus ramucirumab but rather erlotinib plus ramucirumab. It does allow us to consider osimertinib in the second line, but it’s hard to know how to apply these data.
References:
1. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
2. Landre T, Guetz GD, Chouahnia K, et al. Angiogenesis inhibitor plus erlotinib versus erlotinib alone as first-line for advanced non-small cell lung-cancer harboring EGFR mutation. J Clin Oncol. 2020;38(suppl 15):9569. doi:10.1200/JCO.2020.38.15_suppl.9569
3. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625-635. doi:10.1016/S1470-2045(19)30035-X
4. Zhou Q, Wu Y, Cheng Y, et al. Phase 3 study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol. 2019;30(suppl 5):v602-v660. doi:10.1093/annonc/mdz260
5. Nakagawa K, Garon EB, Seto T, et al; RELAY Study Investigators. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655-1669. doi:10.1016/S1470-2045(19)30634-5
6. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study [published correction appears in Lancet Oncol. 2014 Oct;15(11):e475]. Lancet Oncol. 2014;15(11):1236-1244. doi:10.1016/S1470-2045(14)70381-X
7. Stinchcombe TE, J.nne PA, Wang X, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1448-1455. doi:10.1001/jamaoncol.2019.1847
8. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655-1669. doi:10.1016/S1470-2045(19)30634-5
