McKean Uses Updated Data to Support Utilization of Cemiplimab for CSCC Therapy
Treatment options for a 69-year-old patient with poorly differentiated, infiltrative, cutaneous squamous cell carcinoma was discussed by Meredith McKean, MD, MPH and a group of physicians.
Meredith McKean, MD, MPH

Treatment options for a 69-year-old patient with poorly differentiated, infiltrative, cutaneous squamous cell carcinoma (CSCC) was discussed by Meredith McKean, MD, MPH and a group of physicians.
Targeted Oncology™: How would you characterize this patient’s primary lesion on presentation?
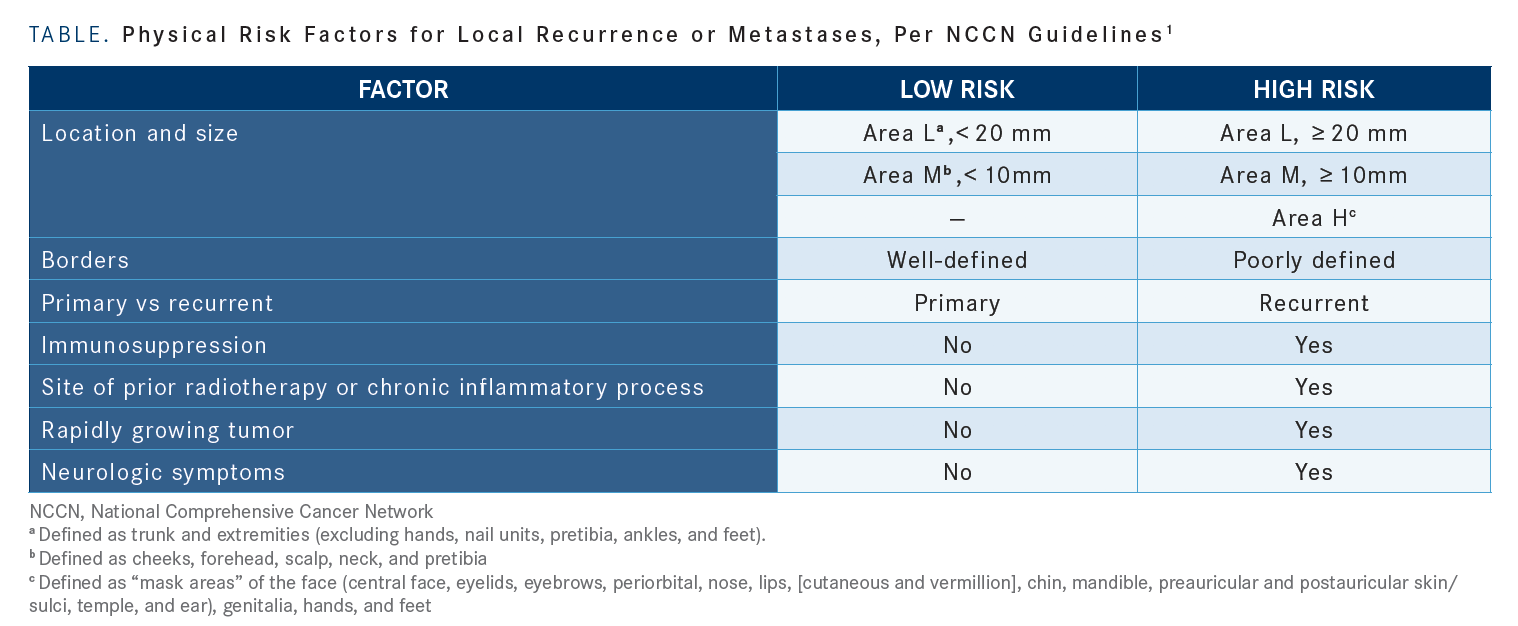
MCKEAN: Looking at the NCCN [National Comprehensive Cancer Network] guidelines for CSCC, for primary disease, there is a helpful chart that gives defining features for patients who are low risk versus high risk for recurrence. Area L is primarily the trunk and extremities, area N would be forehead, scalp, and neck. [Our patient has a] much smaller lesion that’s higher risk on the head and neck versus the trunk and extremities [TABLE].1
Other high-risk features include poorly defined borders if this is already a recurrent disease. Some of the patients that we’ve seen with CSCC are patients who are on immunosuppression, say, for transplants; patients who have had previous radiation to that site, rapidly growing tumors, or neurologic symptoms. Based on the biopsy, the high-risk features are poorly differentiated.
[Other high-risk factors include] depth greater than 6 mm or invasion beyond the subcutaneous fat. And lastly, perineural, lymphatic, and vascular involvements.

What data support the use of cemiplimab in this patient?
The phase 2 EMPOWER-CSCC-1 [NCT02760498] study of cemiplimab recently reported 3-year follow-up data.2 The median follow-up for these patients was just over 1 year, at 15.7 months. There were 3 groups of patients: an initial group of adult patients with metastatic disease; group 2 with locally advanced disease; and group 3, which added more patients with metastatic disease.
Ultimately, the first groups were treated with the weight-based dosing for up to 96 weeks. In group 3, those patients had the flat dosing [of 350 mg] for up to 54 weeks. That will account for some of the discrepancies that we’ll see.
For the inclusion and exclusion criteria, these were, in general, clinical trial candidates who were not eligible for surgery or radiation. There were 193 patients enrolled. Most patients that we’ve seen are of an older population, with a median age of 72 years. For most patients, the primary site of cancer was the head and neck, with about 60% of these being metastatic and 40% being locally advanced. For two-thirds of patients, this was their first line of therapy.
Looking at the response rates for cemiplimab across these populations, the overall response rates by imaging criteria was about 50.8% for that first group, 44.9% for the patients with locally advanced disease, and then 42.9% for the enriched [nodal and/or distant] metastatic population. This was fairly consistent across the populations for a total overall response rate of 46.1%.
Across the populations, there is [a greater than] 10% complete response [CR] rate. The disease control rate is roughly 65% to almost 80% in these populations, with a durable disease control rate and a median observed time to response of just about 2 months.
In general, for immune checkpoint inhibitors, we often think it will be a delayed response. Some of this may be because these are cutaneous lesions that we can also see more easily than on imaging responses, but a fairly impressive response time.
What do the CR data say about the efficacy of the agent?
At the ASCO [American Society of Clinical Oncology] Virtual Scientific Program this year, they showed additional follow-up [and] CR rates. With time, these responses are deepening. In group 1, that’s gone to 2-year follow-up; it’s now up to a 20.3% CR rate. For group 2, it’s been about the same rate of CR at 1 year of follow-up. In group 3, we’re seeing that increase [in CR rate that we saw in group 1]. The median time to initial response is 2 months, but the median time to CR is 11.2 months.2
Is this agent extending survival, as well?
Looking at the PFS [progression-free survival], the median was 18.4 months. Then, the median overall survival still has not been reached in these groups. With PFS, there are overlapping Kaplan-Meier curves, whether it was metastatic or locally advanced disease.
Are there patient factors that can help predict response to cemiplimab?
They’ve done additional analyses, as with other immune checkpoint inhibitors, trying to determine which patients are responding. They tried to stratify patients by their TMB [tumor mutational burden] to see whether they responded. Of the 64% of the samples that they had TMB status for, the median TMB was much higher for responders than nonresponders. I think, clinically, the challenge is that the median is [not] for individual patients; it’s hard to just look at their TMB status and say whether they’re going to respond. It was the same with responders as it was for those with durable disease control.
They also looked at the PD-L1 status. This would be helpful in doing molecular testing. If you had the PD-L1 status, you might be able to see their [likely] objective response. Those patients [with PD-L1 expression ≥ 1%] have a 55% response rate versus 35% in patients with low PD-L1.
Do patients tolerate immunotherapy for CSCC well?
That’s the thing with this patient population, they’re definitely older, more fragile, and managing the disease with minimal toxicity [can be challenging]. With most immune checkpoint inhibitors, almost every patient reports some toxicity.
The most common was fatigue, then diarrhea and nausea. Looking at the grade 3 toxicities or greater, there are usually manageable adverse effects. They have anemia in addition to the fatigue and some other toxicities. I don’t know that I’m necessarily seeing anemia [in my practice]. The thought was attributed to immune checkpoint inhibitors, but it lends to the overlapping toxicities that we see.
What does the future treatment landscape look like for treating CSCC systemically?
There are different ongoing studies using cemiplimab, and a number of studies looking at some of the other PD-1/PD-L1 inhibitors in CSCC.
One of the studies that’s ongoing, and we’re participating in, is an adjuvant study [to determine disease-free survival in patients treated with cemiplimab versus placebo]. Patients who have had resected CSCC have undergone adjuvant radiotherapy with curative intent. These patients are randomized to cemiplimab versus placebo for a year. There’s a strict criterion on the high-risk disease. They have to have extranodal disease, extracapsular extension in transit disease.
References:
1. Clinical Practice Guidelines in Oncology. Bladder cancer, version 4.2019. NCCN. Accessed September 30, 2019. bit.ly/3g9vtvy
2. Rischin D, Khushalani NI, Schmults CD, et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): longer follow-up. J Clin Oncol. 2020;30(suppl 15);10018. doi:10.1200/JCO.2020.38.15_suppl.10018

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More