Alvarez Discusses Bevacizumab Plus Olaparib Combo in BRCA Wild-Type Ovarian Cancer
Ronald D. Alvarez, MD, MBA, weighs the pros and cons of adding bevacizumab to olaparib as treatment of a 69-year-old male patients with BRCA-mutant ovarian cancer.
Ronald D. Alvarez, MD, MBA

Ronald D. Alvarez, MD, MBA, chairman and clinical service chief Betty and Lonnie S. Burnett Professor, Obstetrics and Gynecology, Vanderbilt Medical Center, in Nashville, TN, weighs the pros and cons of adding bevacizumab (Avastin) to olaparib (Lynparza) as treatment of a 69-year-old male patients with BRCA-mutant ovarian cancer.
Targeted Oncology™: What are the advantages and disadvantages of adding bevacizumab (Avastin) to this patient’s treatment regimen?
ALVAREZ: This patient’s current condition is not optimal for debulking. From an efficacy standpoint, she could benefit from bevacizumab, although she has some comorbidities that put her at an increased risk for toxicities, particularly obesity, hypertension, and diabetes. There is also the potential for nephrotoxicity. The cost of treatment might be mitigated now that biosimilars are available.
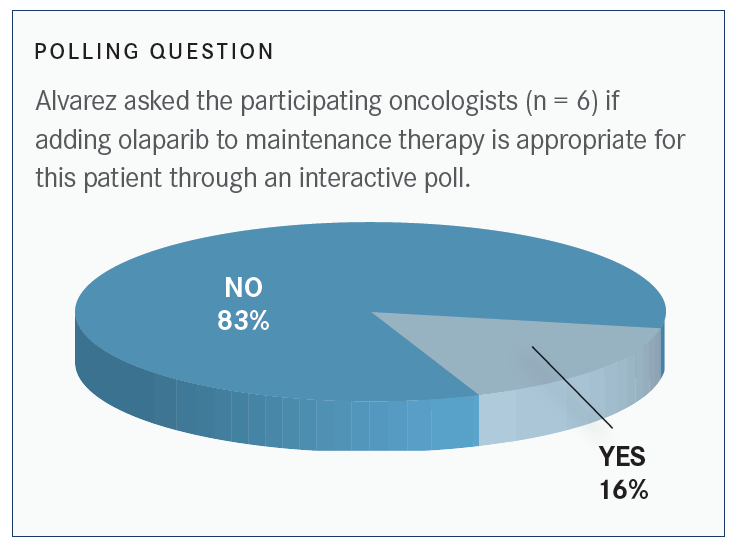
Is olaparib a treatment option here?
This patient receives treatment and goes into a complete remission. She is scheduled to receive bevacizumab maintenance therapy. In this setting, olaparib is not appropriate. Given the information about this patient, her BRCA status and HR [hormone receptor] proficiency, and according to the PAOLA-1 study [NCT02477644],. the addition of olaparib to bevacizumab in patients who are HR positive does not necessarily result in added benefit. It’s not absolutely contraindicated to add the agent, but it doesn’t seem like the evidence would support that.
I think the PAOLA-1 study missed an opportunity to include a treatment arm that evaluated immunotherapy. So the arms could have been chemotherapy, bevacizumab, followed by olaparib only. That might have provided additional information; that is, if you could switch to olaparib only, would the magnitude of benefit be the same as just adding olaparib to Avastin? None of these studies is perfect, and they always miss an opportunity to answer that 1 additional question with 1 additional arm.
How do you define platinum-sensitive versus platinum-resistant recurrent disease?
A couple of years ago, I wrote a paper about redefining the terms platinum sensitive and platinum resistant.2 Early studies involving patients who were platinum sensitive or platinum resistant were conducted when we knew little about the molecular biology of ovarian cancer, and we didn’t know anything about BRCA. In fact, we used a timeline to decide whether somebody was platinum sensitive or platinum resistant.
Now, managing a patient with ovarian cancer requires a continuum [of care], and that makes it a little harder today, particularly in some of these patients, to know when somebody is platinum resistant, other than when they may progress on primary platinum therapy. I find it increasingly hard to decide if somebody is platinum resistant based solely on a timeline.
You have to take in a number of factors that involve the response of primary therapy; the treatment-free interval; the patient’s BRCA status; and, perhaps in some future setting, the patient’s HRD [homologous recombination deficiency] status. I think these factors play an increasing role in whether we abandon platinum-based therapies in patients because we’ve all had patients who we have treated for extended periods. I’ve treated\ patients for 10 years in a row with platinum, and they remained sensitive throughout until the last time we treated them.
Our use of PARP inhibitors will be similar to how we learned how to use bevacizumab. I would think that the learning curve will apply to immuno-oncology agents, too. We started treating patients with platinum-resistant disease, we evaluated the benefit in patients with platinum-sensitive disease, then we looked at its benefit in frontline therapy.
We have some evidence that adding bevacizumab after bevacizumab in the recurrent setting is still beneficial. We just have to assess each individual and figure out if the patient would benefit from bevacizumab in the recurrent setting, even though they may have received bevacizumab up front. I think the same question is going to be asked in clinical trials evaluating PARP inhibitors[in the recurrent setting].
References:
1. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416-2428. doi:10.1056/NEJMoa1911361
2. Alvarez RD, Matulonis UA, Herzog TJ, Coleman RL, Monk BJ, Markman M. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol Oncol. 2016;141(3):405-409. doi:10.1016/j.ygyno.2016.03.005

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More