Therapy Options Compared for the Treatment of Recurrent DLBCL
Loretta J. Nastoupil, MD, discussed the use of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma during a Targeted Oncology Case Based Peer Perspectives event.
Loretta J. Nastoupil, MD

Loretta J. Nastoupil, MD, associate professor, director, Lymphoma, Outcomes Database Section chief, New Drug Development Department of Lymphoma/Myeloma ,Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center, discussed the use of chimeric antigen receptor (CAR) T-cell therapy in patients with diffuse large B-cell lymphoma.
The discussion occurred among a group of oncologist during a Targeted Oncology Case Base Peer Perspective event.
Targeted Oncology™: What clinical trial data support the use of CAR T-cell therapy for this patient?
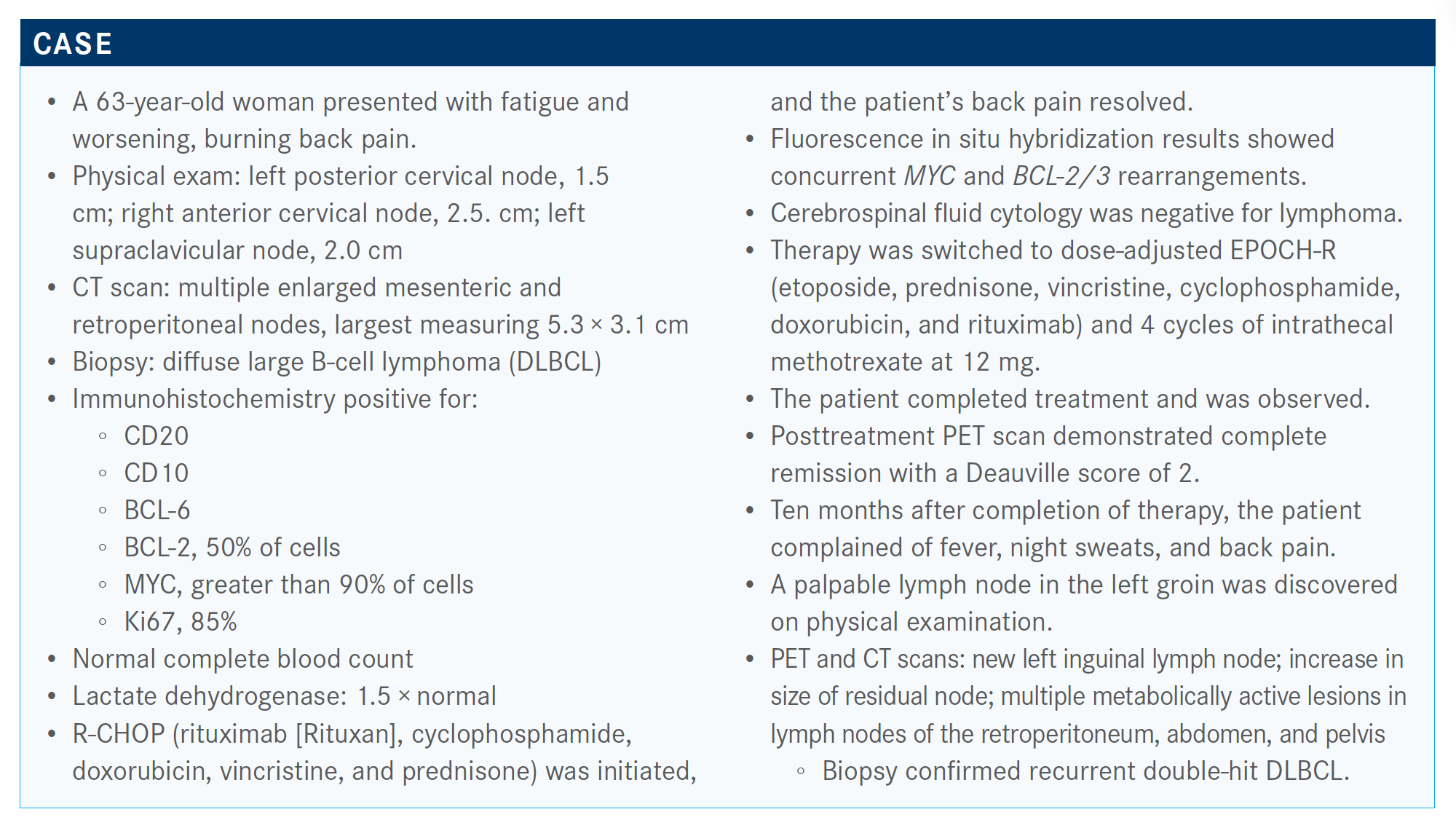
NASTOUPIL: The ZUMA-1 study [NCT02348216] enrolled patients with refractory large cell lymphoma and included about 30% of patients with double-hit features. [It] also included [patients with] primary mediastinal transformed follicular lymphoma. There was a relatively small sample size of 101 patients. This was a single-arm, phase 2 [design]. However, they had a high success rate in terms of enrollment and treatment of patients where 91% of the patients who were enrolled received cell therapy.
ZUMA-1 [examined axicabtagene ciloleucel (axi-cel; Yescarta)], an autologous CD19-directed CAR T-cell therapy. It has a CD28 costimulatory molecule. The lymphocyte depleting regimen is high in this study: 500 mg/m2 of cyclophosphamide and 30 mg/m2 of fludarabine for 3 consecutive days.
The median progress-free survival [PFS] in this study was about 6 months [5.9 months; 95% CI, 3.3-15.0]. The best objective response rate [ORR] was 83% and best complete response [CR] rate was 58%.
There was a stark drop-off and then a flattening of the [Kaplan-Meier PFS] curve. [Experience has shown that] there’s about 40%, to maybe as high as 50%, of patients who can be cured with this, but it’s hard to identify who they are. The patients who don’t respond generally do poorly within the first 3 to 6 months.
The JULIET study [NCT02445248] was a bit different. It’s a larger multicenter study [that examined tisagenlecleucel (Kymriah)]. It was a much longer time from enrollment to infusion of CAR T cells; as a result, bridging therapy was allowed. They also included relapsed, not just refractory, patients, and they excluded patients with primary mediastinal disease.2
There [were] lower response rates [in] this study population of 52%. The CR rate, though, is notable at 40%. The patients who had CR had durability of that CR. About 40% of the patients had a meaningful outcome with this CAR-T cell therapy, which was a 4-1BB construct. This is an autologous [product] with a CD19 target.
The efficacy is hard to compare across the studies because they were different in terms of patient eligibility and trial conduct. However, my general sense is that the vast majority of patients have about a 40%, to maybe as high as 50%, meaningful CR that is durable, and the overall survival [OS] is quite notable. With ZUMA-1, the median OS settles in at 27.1 months.
Are there any CAR T-cell therapies that may be added to the list of available products for these patients?
Liso-cel [lisocabtagene maraleucel] is the third [CAR T-cell product that is] anticipated for FDA approval. That’s also a 41BB construct similar to tisagenlecleucel. It’s different in that there’s a fixed CD4 to CD8 ratio and a longer time for manufacturing in comparison to axi-cel. But again, there is meaningful efficacy.3
How does the toxicity compare between these agents?
The toxicity looks to be different, but different grading systems were applied in the studies. When you apply the same rating system retrospectively, patients have fewer grade 3 or higher cytokine release syndrome in the JULIET study than what was initially reported.4
Axi-cel tends to have the highest rate of grade 3 or higher neurotoxicity and liso-cel tends to have the most favorable toxicity profile. Though because it’s the third [agent to become available], we’ve [become] much better at identifying and mitigating some of these acute toxicities.
There are differences, in my opinion, across the 3 constructs in terms of safety, despite the efficacy being quite similar.
Why are these data important to review?
We know CAR-T cell therapy has transformed outcomes for about 40% of patients; however, it’s logistically challenging....We’ve struggled with identifying what makes a good CAR-T cell candidate outside of having chemotherapy-refractory disease and being in a third-line setting.
What other therapy options could be used in this patient if she is not a candidate for CAR T-cell therapy?
Polatuzumab vedotin [Polivy] in combination with bendamustine and rituximab [BR] was approved based on a randomized phase 2 study [NCT02257567].5 Of note, this was a study that included both follicular and large cell lymphoma. However, the follicular [cohort showed] a negative result. There was no significant impact with the addition of polatuzumab vedotin to BR, which is not necessarily surprising given that bendamustine is a very active agent for follicular lymphoma.
It is not an active agent in large cell lymphoma. One of the reasons why it was included in this study design is because the potential for challenge with polatuzumab was identifying an agent in third-line large cell lymphoma setting where you wouldn’t have additive.
Please describe the trial that led to the approval of polatuzumab vedotin.
For baseline characteristics, and this is specifically looking at the patients with large cell lymphoma, the median age was 67 years [range, 33-86] in the polatuzumab/BR arm versus 71 years [range, 30-84] in the BR-only arm. In terms of median number of prior lines of therapy, it was 2 for both arms. In terms of prior stem cell transplant, about 25% had undergone transplant in the polatuzumab-BR arm versus 15% in the BR-arm.
ORR by independent review with the polatuzumab-BR was much higher versus the BR-only arm [45% versus 17.5%].6
Importantly, the median PFS was significantly different. If patients received polatuzumab, the median PFS was 9.5 months versus 3.7 months in the control arm [0.36; 95% CI, 0.21-0.63; P < .001].
The important message is that there weren’t any subgroups that benefited from BR alone, and this included criteria such as bulky disease, number of prior lines of therapy, and duration of response with the prior line of therapy.
The OS was significantly better if you had polatuzumab plus BR, with a median of 12.4 months versus 4.7 months [HR, 0.42; 95% CI, 0.24-0.75; P = .002]. Similarly, there were no subgroups that tended to benefit from receiving BR alone.
What was the toxicity profile of this regimen for patients?
[It is] important to note that peripheral neuropathy, which is an adverse event associated with this antibody-drug conjugant, was mostly reported as grade 1 events. [The effect was] slightly additive if you had the BR and polatuzumab versus no polatuzumab.
In terms of neutropenia, grade 3/4 events with the combination occurred in 46.2% of patients, and that’s higher when compared with BR alone [33.3%]. However, febrile neutropenia events were no different between arms [10.3% vs 12.8%].
References:
1. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. doi:10.1056/NEJMoa1707447
2. Schuster SJ, Bishop MR, Tam CS, et al; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. doi:10.1056/NEJMoa1804980
3. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. doi:10.1016/S0140-6736(20)31366-0
4. Abramson JS. Anti-CD19 CAR T-cell therapy for B-cell non-Hodgkin lymphoma. Transfus Med Rev. 2020;34(1):29-33. doi:10.1016/j.tmrv.2019.08.003
5. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed November 13, 2020. https://bit.ly/2IBZGrv
6. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More